Abstract
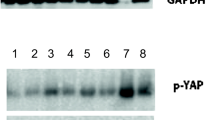
The aim of this study was to characterize phenotypic alterations along the progression of breast carcinoma from primary tumor to pleural effusion through analysis of the expression of nerve growth factor (NGF) and its receptors phospho-TrkA (p-TrkA activated receptor) and p75. Sections from 42 malignant pleural effusions from breast cancer patients and 65 corresponding solid tumors (34 primary, 31 metastatic) were evaluated for protein expression of the activated p-TrkA receptor. The majority of lesions were additionally studied for NGF and p75 expression. Six effusions and four breast carcinoma cell lines were studied for expression of p-TrkA using immunoblotting (IB). Membrane expression of p-TrkA was high in carcinoma cells in effusions (39/42, 93%) and locoregional recurrences (12/13, 92%), with significantly lower expression in both primary tumors (14/34, 41%) and lymph node metastases (8/18, 44%), respectively (p < 0.001 for effusions vs. primary tumors; p = 0.001 for effusions vs. lymph nodes). In contrast, p75 expression was less frequent in effusions compared to both primary tumors and lymph node metastases, significantly so for the latter (p = 0.019). NGF expression was comparable at all sites, but its expression in tumor cells in effusions (7/21 cases) was limited to cases in which time to progression (TTP) to effusion occurred within 5 years or less from primary operation. In univariate analysis of survival, mean and median TTP were 6.3 and 6 years for NGF-negative effusions, compared to 3 and 4 years for NGF-positive cases (p = 0.013). IB confirmed expression of p-TrkA in five of six effusions, while all four breast cancer cell lines were p-TrkA-negative. Our data provide the first documented evidence of molecular events that occur along tumor progression of breast carcinoma from primary tumors to effusion. The almost universal expression of p-TrkA in cancer cells in effusions and late recurrences is in full agreement with our recent report linking this factor with poor prognosis in ovarian cancer. Furthermore, the rapid progression to effusion in cases showing NGF expression in tumor cells underscores the aggressive clinical behavior of tumors that are able to utilize this pathway in an autocrine manner.
Similar content being viewed by others
References
Barbacid M: Neurotrophic factors and their receptors. Curr Opin Cell Biol 7: 148–155, 1995
Kaplan DR, Miller FD: Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol 9: 213–221, 1997
Patapoutian A, Reichardt LF: Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2001
Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM: Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem 268: 15150–15157, 1993
Weismann C, Ultsch MH, Bass SH, de Vos AM: Crystal structure of the nerve growth factor in complex with the ligandbinding domain of the TrkA receptor. Nature 401: 184–188, 1999
Greene LA, Kaplan DR: Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opin Neurobiol 5: 579–587, 1995
Kaplan DR, Miller FD: Neutrophin signal transduction in the nervous system. Curr Opin Neurobiol 10: 381–391, 2000
Rudkin BB, Lazarovici P, Levi BZ, Abe Y, Fugita K, Guroff G: Cell cycle-specific action of nerve growth factor in PC12 cells: differentiation without proliferation. EMBO J 11: 3319–3325, 1989
Urdiales JL, Becker E, Andrieu M, Thomas A, Jullien J, van Grunsven LA, Menut S, Evan GI, Martin-Zanca D, Rudkin BB: Cell cycle phase-specific surface expression of nerve growth factor receptors TrkA and p75NTR. J Neurosci 17: 6767–6775, 1998
Gollapudi L, Neet KE: Different mechanisms for inhibition of cell proliferation via cell cycle proteins in PC12 cells by nerve growth factor and staurosporine. J Neurosci Res 49: 461–474, 1997
Billon N, van Grunsven LA, Rudkin BB: The CDK inhibitor p21WAF1 / Cip1 is induced through a p300-dependent mechanism during NGF-mediated neuronal differentiation of PC12 cells. Oncogene 13: 2047–2054, 1996
Montano X: p53 associates with trk tyrosine kinase. Oncogene 15: 245–246, 1997
Brown A, Browes C, Mitchell M, Montano X: c-abl is involved in the association of p53 and trk A. Oncogene 19: 3032–3040, 2000
Nakagawara A: Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 169: 107–114, 2001
Shibayama E, Koizumi H: Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 148: 1807–1818, 1996
McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD: Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA 96: 4540–4545, 1999
Katzir I, Shani J, Shabashov D, Dagan J, Lazarovici P: Establishment and characterization of pheochromocytoma tumor model expressing different levels of trkA receptors. Cancer Lett (in press)
Miknyoczki SJ, Wan W, Chang H, Dobrzanski P, Ruggeri BA, Dionne CA, Buchkovich K: The neurotrophin-Trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma in nude mice. Clin Cancer Res 8: 1924–1931, 2002
Nakagawara A, Arima M, Azar CG, Scavarda NJ, Brodeur GM: Inverse relationship between trk expression and Nmyc amplification in human neuroblastomas. Cancer Res 52: 1364–1368, 1992
Suzuki E, Bogenmann H, Shimada D, Stram RC, Seeger RC: Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst 85: 377–384, 1993
Fanburg-Smith JC, Miettinen M: Low-affinity nerve growth factor receptor (p75) in dermatofibrosarcoma protuberans and other nonneuronal tumors: a study of 1,150 tumors and fetal and adult normal tissues. Hum Pathol 32: 976–983, 2001
Krygier S, Djakiew D: Neurotrophin receptor p75NTR suppresses growth and nerve growth factor-mediated metastasis of human prostate cancer cells. Int J Cancer 98: 1–7, 2002
Pflug B, Djakiew D: Expression of p75NTR in human prostate epithelial tumor cell line reduces nerve growth factor-induced cell growth by activation of programmed cell death. Mol Carcinog 23: 106–114, 1998
Pflug BR, Onoda M, Lynch JH, Djakiew D: Reduced expression of the low affinity nerve growth factor receptor in benign and malignant human prostate tissue and loss of expression in four human metastatic prostate tumor cell lines. Cancer Res 52: 5403–5406, 1992
MacGrogan D, Saint-Andre' JP, Dicou E: Expression of nerve growth factor and nerve growth factor receptor genes in human tissues and in prostatic adenocarcinoma cell lines. J Neurochem 59: 1381–1391, 1992
Davidson B, Lazarovici P, Ezersky A, Nesland JM, Berner A, Risberg B, Trope' CG, Kristensen GB, Goscinski M, van de Putte G, Reich R: Expression levels of the NGF receptors TrkA and p75 in effusions and solid tumors of serous ovarian carcinoma patients. Clin Cancer Res 7: 3457–3464, 2001
Davidson B, Reich R, Lazarovici P, Nesland JM, Risberg B, Trope' CG, Flørenes VA: Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res 9: 2248–2259, 2003
Greenlee RT, Hill-Harmon MB, Murray T, Thun M: Cancer statistics, 2001. CA Cancer J Clin 51: 15–36, 2001
Hausheer FH, Yarbro JW: Diagnosis and treatment of malignant pleural effusion. Semin Oncol 12: 54–75, 1985
Martinez-Moragon E, Aparicio J, Sanchis J, Menendez R, Cruz Rogado M, Sanchis F: Malignant pleural effusion: prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration 65: 108–113, 1998
Wilkes JD, Fidias P, Vaickus L, Perez RP: Malignancy-related pericardial effusion. 127 cases from the Roswell Park Center Institute. Cancer 76: 1377–1387, 1995
Dieterich M, Goodman SN, Rojas-Corona RR, Emralino AB, Jimenez-Joseph D, Sherman ME: Multivariate analysis of prognostic features in malignant pleural effusions from breast cancer patients. Acta Cytol 38: 945–952, 1994
Banerjee AK, Willetts I, Robertson JF, Blamey RW: Pleural effusion in breast cancer: a review of the Nottingham experience. Eur J Surg Oncol 20: 33–36, 1994
Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H: Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 22: 5592–5601, 2003
Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H: Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem 273: 16659–16662, 1998
Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H: Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem 276: 17864–17870, 2001
El Yazidi-Belkoura I, Adriaenssens E, Dolle L, Descamps S, Hondermarck H: Tumor necrosis factor receptor-associated death domain protein is involved in the neurotrophin receptor-mediated antiapoptotic activity of nerve growth factor in breast cancer cells. J Biol Chem 278: 16952–16956, 2003
Chiarenza A, Lazarovici P, Lempereur L, Cantarella G, Bianchi A, Bernardini R: Tamoxifen inhibits nerve growth factor-induced proliferation of the human breast cancerous line MCF-7. Cancer Res 61: 3002-3008
Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP: Expression of nerve growth factor receptors and prognostic value in human breast cancer. Cancer Res 61: 4337–4340, 2001
Aragona M, Panetta S, Silipigni AM, Romeo DL, Pastura G, Mesiti M, Cascinu S, Torre F: Nerve growth factor receptor immunoreactivity in breast cancer patients. Cancer Invest 19: 692–697, 2001
Davidson B, Risberg B, Kristensen G, Kvalheim G, Emilsen E, Bjåmer A, Berner A: Detection of cancer cells in effusions from patients diagnosed with gynecological malignancies-evaluation of five epithelial markers. Virchows Arch 435: 43–49, 1999
Davidson B, Nielsen S, Christensen J, Asschenfeldt P, Berner A, Risberg B, Johansen P: The role of Desmin and N-cadherin in effusion cytology. A comparative study using established markers of mesothelial and epithelial cells. Am J Surg Pathol 25: 1405–1412, 2001
Hochman J, Park SS, Lazarovici P, Bergel M, Gottesman MM: Monoclonal antibodies to immunogenic lymphoma cell variants displaying impaired neoplastic properties: characterization and applications. J Natl Cancer Inst 82: 1821–1826, 1990
Katzir I, Shani J, Regev K, Shabashov D, Lazarovici P: A quantitative bioassay for nerve growth factor, using PC12 clones expressing different levels of trkA receptors. J Mol Neurosci 18: 251–264, 2002
Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, Isaacs JT: Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res 7: 2237–2245, 2001
Segal RA, Greenberg ME: Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19: 463–489, 1996
Roux PP, Bhakar AL, Kennedy TE, Barker PA: The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem 276: 23097–23104, 2001
Calza L, Giardino L, Guiliani A, Aloe L, Levi-Montalcini R: Nerve growth factor control of neuronal expression of angiogenic and vasoactive factors. Proc Natl Acad Sci USA 98: 4160–4165, 2001
Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R: Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J 16: 1307–1309, 2002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidson, B., Reich, R., Lazarovici, P. et al. Altered Expression and Activation of the Nerve Growth Factor Receptors TrkA and p75 Provide the First Evidence of Tumor Progression to Effusion in Breast Carcinoma. Breast Cancer Res Treat 83, 119–128 (2004). https://doi.org/10.1023/B:BREA.0000010704.17479.8a
Issue Date:
DOI: https://doi.org/10.1023/B:BREA.0000010704.17479.8a