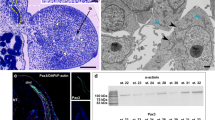
Abstract
The initial stages of myogenesis going in myoblasts include the stages of induction, determination, and differentiation. The induction and determination of cells in the myotomes are controlled by morphogenetic signals from neighboring tissues of the notochord and neural tube manifested as expression of genes of Shh and Wnt families, respectively. In fish (at the example of danio), this signal is passed to somite cells neighboring the notochord; later the cells migrate to the embryo surface and differentiate into slow muscle fibers. Synthesis of the main contractile proteins, primarily the components of myosin molecule—heavy chain (MHC) and individual isoforms of light chains (MLC1, MLC2, and MLC3)—are encoded by different genes during different ontogenetic stages. The peptide maps obtained after α-chymotrypsin digestion of MHCs from larvae, fast and slow skeletal muscle of loach are different, which points to differences in their primary structure. In addition, considerable differences were revealed in the structure of MLC isoforms at different ontogenetic stages. The definitive fast muscle contained three light chain types, MLC1, MLC2, and MLC3; slow muscle, MLC1 and MLC3; while the larval muscle fibers included a specific larval MLCL in addition to MLC3.
Similar content being viewed by others
REFERENCES
Adams, G.R., McCue, S.A., Zeng, M., et al., Timecourse of Myosin Heavy Chain Transitions in Neonatal Rats: Importance of Innervation and Thyroid State, Am. J. Physiol., 1999, vol. 276, pp. 954-961.
Bandman, E., The Avian Myosin Heavy Chains, Gene Expression in Neuromuscular Development, Blau, H. and Kelly, A.M., Eds., New York: Raven, 1992, pp. 68-82.
Barton, P. and Buckingham, M.E., The Myosin Alkali Light Chain Proteins and Their Genes, Biochem. J., 1985, vol. 231, pp. 249-257.
Bernstein, S.I., Hansen, C.J., Becker, K.D., et al., Alternative RNA Splicing Generates Transcripts Encoding a Thorax-Specific Isoform of Drosophila melanogaster Myosin Heavy Chain, Mol. Cell. Biol., 1986, vol. 6, pp. 2511-2519.
Blagden, C.S., Currie, P.D., Ingham, P.W., et al., Notochord Induction of Zebrafish Slow Muscle Mediated by Sonic Hedgehog, Genes Devel., 1997, vol. 11, 2163-2175.
Bober, E., Brand-Soberi, B., Ebensperger, C., et al., Initial Steps of Myogenesis in Somites Are Independent of Influence from Axial Structures, Development, 1994, vol. 120, pp. 3073-3082.
Braun, T., Bober, E., Winter, B., et al., Myf-6, a New Member of the Human Gene Family of Myogenic Determination Factors: Evidence for a Gene Cluster on Chromosome 12, EMBO J., 1990, vol. 9, pp. 821-829.
Braun, T., Buschhausen, D.C., Bober, E., et al., A Novel Human Muscle Factor Related to but Distinct from MyoD1 Induced Myogenic Conversion in 10T1/2 Fibroblasts, EMBO J., 1989, vol. 8, pp. 701-710.
Breitbart, R.E., Nguyen, H.T., Medford, R.M., et al., Intricate Combinatorial Patterns of Exon Splicing Generate Multiple Regulation Troponin T Isoforms from a Single Gene, Cell, 1985, vol. 41, pp. 67-82.
Butler-Browne, G.S. and Whalen, R.C., Myosin Isozyme Transitions Occurring during the Postnatal Development of the Soleus Muscle, Dev. Biol., 1984, vol. 102, pp. 324-332.
Christ, B., Brand-Saberi, B., Grim, M., et al., Local Signaling in Dermomyotomal Cell Type Specification, Anat. Embryol., 1992, vol. 186, pp. 505-510.
Collier, V.L., Kronert, W.A., O'Donnell, T.O., et al., Alternative Myosin Hinge Regions Are Utilized in a Tissue-Specific Fashion That Correlates with Muscle Contraction Speed, Genes Devel., 1990, vol. 4, pp. 885-895.
Crow, M.T., Olson, P.S., and Stockdale, F.E., Myosin Light Chain Expression during Avian Muscle Development, J. Cell Biol., 1983, vol. 96, pp. 736-744.
Dalla Libera, L., Carpene, E., Theibert, J., et al., Fish Myosin Alkali Light Chains Originate from Two Different Genes, J. Muscle Res. Cell Motil., 1991, vol. 12, pp. 366-371.
Davis, R.L., Weintraub, H., and Lassar, A.B., Expression of a Single Transfected cDNA Converts Fibroblasts to Myoblasts, Cell, 1987, vol. 51, pp. 987-1000.
Devoto, S.H., Melancon, E., Eisen, J.S., et al., Identification of Separate Slow and Fast Muscle Precursor Cells in vivo, prior to Somite Formation, Development, 1996, vol. 122, pp. 3371-3380.
Dhoot, G.K., Selective Synthesis and Degradation of Slow Skeletal Myosin Heavy Chains in Developing Muscle Fibers, Muscle and Nerve, 1986, vol. 9, pp. 155-164.
Edmonson, D.G. and Olson, E.N., A Gene with Homology to the myc Similarity Region of MyoD1 Is Expressed during Myogenesis and Is Sufficient to Activate the Muscle Differentiation Program, Genes Devel., 1989, vol. 3, pp. 628-640.
Emerson, C.P., Skeletal Myogenesis: Genetics and Embryology of the Fore, Curr. Opin. Genet. Devel., 1993, vol. 3, pp. 265-274.
Focant, B., Huriaux, F., Vandewalle, P., et al., Myosin, Parvalbumin and Myofibril Expression in Barbel (Barbus barbus L.) Lateral White Muscle during Development, Fish Physiol. Biochem., 1992, vol. 10, pp. 133-143.
Frank, D. and Harland, R., Transient Expression of XMyoD in Nonsomatic Mesoderm of Xenopus Gastrulae, Development, 1991, vol. 113, pp. 1387-1393.
Frank, D. and Weeds, A.G., The Amino-Acid Sequences of the Alkali Light Chains of Rabbit Skeletal-Muscle Myosin, Eur. J. Biochem., 1974, vol. 44, pp. 317-334.
Gauthier, C.F., Lowey, S., Bormioli, P.A., et al., Distribution and Properties of Myosin Isozymes in Developing Avian and Mammalian Skeletal Muscle Fibres, J. Cell Biol., 1982, vol. 92, pp. 471-484.
George, E.L., Ober, M.B., and Emerson, C.P., Jr., Functional Domains of the Drosophila melanogaster Muscle Myosin Heavy Chain Gene Are Encoded by Alternative Spliced Exons, Mol. Cell Biol., 1989, vol. 9, pp. 2957-2974.
Goulding, M., Lumsden, A., and Paquette, A.J., Regulation of Pax3 Expression in the Dermomyotome and Its Role in Muscle Development, Development, 1994, vol. 120, pp. 957-971.
Hirayama, Y., Kantoh, S., Nakaya, M., et al., The Two Essential Light Chains of Carp Fast Skeletal Myosin, LC1 and LC3, Are Encoded by Distinct Genes and Change Their Molar Ratio Following Temperature Acclimation, J. Exp. Biol., 1997, vol. 200, pp. 693-701.
Hoh, J.F.Y. and Yeoh, G.P.S., Rabbit Skeletal Myosin Isoenzymes from Foetal, Fast-Twitch and Slow-Twitch Muscles, Nature, 1979, vol. 280, pp. 321-323.
Holland, L.Z., Pace, D.A., Blink, M.L., et al., Sequence and Expression of Amphioxus Alkali Myosin Light Chain (amphiMLC-Alk) throughout Development: Implications for Vertebrate Myogenesis, Dev. Biol., 1995, vol. 171, pp. 665-676.
Hopwood, N.D., Pluck, A., Gurdon, J.B., et al., Expression of XMyoD1 Protein in Early Xenopus laevis Embryos, Development, 1992, vol. 114, p. 31.
Hughes, S.M., Blagden, C.S., Li, X., et al., The Role of Hedgehog Proteins in Vertebrate Slow and Fast Skeletal Muscle Pattering, Acta Physiol. Scand., 1998, vol. 163, pp. 7-12.
Huriaux, F., Vandewalle, P., Philippart, J.C., et al., Electrophoretic Comparison of Myosin Light Chains and Parvalbumins of Trunk and Head Muscles from Two Barbel (Barbus barbus) Populations, Comp. Biochem. Physiol. B. Comp. Biochem., 1990, vol. 97, pp. 547-553.
Kalamkarova, M.B., Kofman, E.V., and Aleinikova, K.S., Myosin Isoforms of Skeletal Muscles in Development, Ontogenez, 1987, vol. 18, p. 573.
Kazanskaya, O.V., Pal'mbakh, L.R., and Ozernyuk, N.D., Development of Skeletal Muscle in Teleost Fish, Zh. Obshch. Biol., 1986, vol. 47, pp. 656-666.
Kenny-Mobbs, T. and Thorogood, P., Autonomy of Differentiation in Avian Brachial Somites and the Influences of Adjacent Tissues, Development, 1987, vol. 100, pp. 449-462.
Lowey, S., Benfiels, P.A., Le Blanc, D.D., et al., Characterization of Myosin from Embryonic and Development: Molecular and Cellular Control, New York: Cold Spring Harbor Lab., 1982, pp. 15-24.
Lyons, G.E., Haselgrove, J., Kelly, A.M., et al., Myosin Transitions in Developing Fast and Slow Muscles of the Cat Hind Limb, Differentiation, 1983, vol. 25, pp. 168-175.
Matsuda, R., Maita, T., Kato, Y., et al., Amino Acid Sequence of the Cardiac L-2A, L-2B and Gizzard 17 000 Light Chains of Chicken Muscle Myosin, FEBS Lett., 1981, vol. 135, pp. 232-236.
Moutou, K.A., Canario, A.V.M., Mamuris, Z., et al., Molecular Cloning and Sequence of Sparus aurata Skeletal Myosin Light Chains Expressed in White Muscle: Developmental Expression and Thyroid Regulation, J. Exp. Biol., 2001, vol. 204, pp. 3009-3018.
Munsterberg, A.E. and Lassar, A.B., Combinatorial Signals from the Neural Tube, Floor Plate and Notochord Induce Myogenic bHLH Gene Expression in the Somite, Development, 1995, vol. 121, pp. 651-660.
Munsterberg, A.E., Kitajewski, J., Bumcrot, D.A., et al., Combinatorial Signaling by Sonic Hedgehog and Wnt Family Members Induces Myogenic bHLH Gene Expression in the Somite, Genes Devel., 1995, vol. 9, pp. 2911-2922.
Nabeshima, Y., Fujii-Kuriyama, Y., Mura-Matsu, M., et al., Alternative Transcription and Two Modes of Splicing Result in Two Myosin Light Chains from One Gene, Nature, 1984, vol. 308, pp. 333-338.
Nareiko, V.G., Myosin Isoforms of the Developing Skeletal Muscles in the Loach, Ontogenez, 1988, vol. 19, pp. 601-606.
Nareiko, V.G. and Ozernyuk, N.D., Differences in the Composition of Contractile Proteins from Red and White Skeletal Muscles of Teleost Fishes, Dokl. Akad. Nauk SSSR, 1988, vol. 296, pp. 1022-1024.
Nguen, H.T., Gubits, R.M., Wydro, R.M., et al., Sarcomeric Myosin Heavy Chain Is Coded by a Highly Conserved Multigene Famity, Proc. Natl. Acad. Sci. USA, 1982, vol. 79, 5230-5234.
O'Farrell, P., High Resolution Two-Dimensional Electrophoresis of Proteins, J. Biol. Chem., 1975, vol. 250, pp. 4007-4021.
Oakley, B.R., Kirsch, D.R., and Morris, N.R., A Simplified Ultrasensitive Silver Stain for Detecting Proteins in Polyacrylamide Gels, Anal. Biochem., 1980, vol. 105, pp. 361-363.
Olson, E.N., MyoD Family: A Paradigm for Development?, Genes Devel., 1990, vol. 4, pp. 1454-1461.
Olson, E.N. and Klein, W.H., bHLH Factors in Muscle Development: Dead Lines and Commitments, What to Leave in and What to Leave Out, Genes Devel., 1994, vol. 8, pp. 1-8.
Ozernyuk, N.D., Regulation of Myogenesis, Izv. Akad. Nauk. Ser. Biol, 1998, no. 3, pp. 330-343.
Periasamy, M., Wieczorek, D.F., and Nadal-Ginard, B., Characterization of a Developmentally Regulated Perinatal Myosin Heavy Chain Gene Expressed in Skeletal Muscle, J. Biol. Chem., 1984, vol. 259, pp. 13 573-13 578.
Rhodes, S.J. and Kenieszny, S.F., Identification of MRF4: A New Member of the Muscle Regulatory Factor Gene Family, Genes Devel., 1989, vol. 3, pp. 2050-2056.
Robert, B., Daubas, P., Akimenko, M.-A., et al., A Single Locus in the Mouse Encodes Both Myosin Light Chains 1 and 3, A Second Locus Corresponds to a Related Pseudogene, Cell, 1984, vol. 39, pp. 129-140.
Rong, P.M., Teillet, M.A., Ziller, C., et al., The Neural Tube/Notochord Complex Is Necessary for Vertebral but Not Limb and Body Wall Striated Muscle Differentiation, Development, 1992, vol. 115, pp. 657-672.
Rozek, C.E. and Davidson, N., Differential Processing of RNA Transcribed from Single-Copy Drosophila Myosin Heavy Chain Gene Produces Four mRNA That Encode Two Polypeptides, Proc. Natl. Acad. Sci. USA, 1986, vol. 83, pp. 2128-2132.
Takano-Ohmuro, H., Obinata, T., Masaki, T., et al., Changes in Myosin Isozymes during Development of Chicken Breast Muscle, J. Biochem., 1982, vol. 91, pp. 11 305-11 309.
Teillet, M.A. and LeDouarin, N.M., Consequences of Neural Tube and Notochord Excision on the Development of the Peripheral Nervous System in the Chick Embryos, Dev. Biol., 1983, vol. 98, pp. 192-211.
Thiebaud, P., Rescan, P.-Y., Barillot, W., et al., Developmental Program Expression of Myosin Alkali Light Chain and Skeletal Actin Genes in Rainbow Trout Oncorhynchus mykiss, Biochim. Biophys. Acta, 2001, vol. 1519, pp. 139-142.
Van Raamsdonk, W., Pool, C.W., and te Kronnie, G., Differentiation of Muscle Fibre Types in the Teleost Brachydanio rerio, Anat. Embryol., 1978, vol. 153, pp. 137-155.
Weeds, A.G. and Lowey, S., Substructure of the Myosin Molecule. II. The Light Chains of Myosin, J. Mol. Biol., 1971, vol. 61, pp. 701-725.
Weinberg, E.S., Allende, M.L., Kelly, C.S., et al., Developmental Regulation of Zebrafish MyoD in Wild-Type, No Tail and Spadetail, Development, 1996, vol. 122, pp. 271-280.
Weintraub, H., Davis, R., Tapscott, S., et al., The MyoD Gene Family: Nodal Point during Specification of the Muscle Cell Lineage, Science, 1991, vol. 251, pp. 761-764.
Whalen, R.G., Butler-Browne, G.S., and Gros, F., Identification of a Novel Form of Myosin Light Chain Present in Embryonic Muscle Tissue and Cultured Muscle Cells, J. Mol. Biol., 1978, vol. 126, pp. 415-431.
Whalen, R.G., Sell, S.M., Butler-Browne, G.S., et al., Three Myosin Heavy Chain Isoenzymes Appear Sequentially in Rat Muscle Development, Nature, 1981, vol. 292, pp. 805-809.
Winkelmann, D.A., Lowey, S., and Press, J.L., Monoclonal Antibodies Localize Changes on Myosin Heavy Chains Isozymes during Avian Myogenesis, Cell, 1983, vol. 34, pp. 295-306.
Xu, Y., He, J., Lim, T.M., et al., Asynchronous Activation of 10 Muscle-Specific Protein (MSP) Genes during Zebrafish Somitogenesis, Devel. Dyn., 2000, vol. 219, pp. 201-215.
Xu, Y., He, J., Tian, H.L., et al., Fast Skeletal Muscle-Specific Expression of a Zebrafish Myosin Light Chain 2 Gene and Characterization of Its Promoter by Direct Injection into Skeletal Muscle, DNA Cell Biol., 1999, vol. 18, pp. 85-95.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozernyuk, N.D., Nareiko, V.G., Smirnova, Y.A. et al. Pattern of Skeletal Muscle Differentiation in Fish: Molecular Biological Approaches. Biology Bulletin 31, 209–215 (2004). https://doi.org/10.1023/B:BIBU.0000030139.70372.73
Issue Date:
DOI: https://doi.org/10.1023/B:BIBU.0000030139.70372.73