Abstract
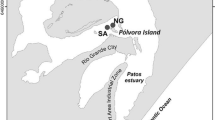
Measurements of O2, Fe(II), Mn(II)and HS5 in salt marshsediments in the Tagus Estuary, Portugal, made with a voltammetric microelectrode, reveal strong seasonal differences in pore water composition within the 20~cm deep root zone. In spring, oxygen was below detection limit except close to the sediment surface. Fe(II) was present below 5 cm in concentrations ranging from detection limit to 1700 μM. In summer, oxygen was present in the pore water almost to the bottom of the root zone in concentrations ranging from detection limit to more than 100 μM. The spatial variability was intense: O2 concentrations as high as 78 μM and as low as 25 μM existed within 2~mm of each other. Fe(II) was below detection limit except towards the bottom of the root zone. In late fall, oxygen was found to 8 cm depth, but in concentrations lower than in summer, and Fe(II) was present below 9 cm. Mn(II) was found at levels declining from typical values of 200 μM in spring to less than 20 μM in late fall. With one exception, sulfide was below the detection limit in all measurements. During periods when dissolved Fe(II) is available in the pore water at the same time as 2 is delivered by roots, iron-rich concretions can form on roots. These conditions, which lead to precipitation of iron oxide in the sediment adjacent to roots, exist in spring, when new roots infiltrate anoxic Fe(II) containing sediment. They do not exist in summer, when dissolved Fe(II) is unavailable, or in winter, when oxygen is unavailable. The seasonal redox pattern revealed by the pore water chemistry is driven by the annual cycle of growth and decay of roots.
Similar content being viewed by others
References
Allaway, W. G., Curran, M., Hollington, L. M., Ricketts, M. C., and Skelton, N. J. (2001) Gas space and oxygen exchange in roots of Avicennia marina (Forrsk.) Vierh. var. australasica (Walp.) ex N.C. Duke, the Grey Mangrove, Wetlands Ecology and Management 9, 211–2001.
Anderson, C. E. (1974) A review of structure in several North Carolina salt marsh plants, In: Ecology of Halophytes (eds. R. J. Reimold and W. H. Queen), Academic Press, pp. 307–344.
Anschutz, P., Zhong, S., Sundby, B., Mucci, A., and Gobeil, C. (1998) Burial efficiency of phosphorus and the geochemistry of iron in continental margin sediments, Limnology & Oceanography 43(1), 53–64.
Armstrong, W. (1978) Root aeration in the wetland condition, In: Plant Life in Anaerobic Environments (eds. D. D. Hook and C. R. M. M. Crawforth), Ann Arbor Science Publishers, pp. 269–298.
Bertness, M. D. (1985) Fiddler crab regulation of Spartina alterniflora production on a New England saltmarsh, Ecology 66, 1042–1055.
Bianchi, T. S. and Findlay, S. (1991) Decomposition of Hudson River macrophytes: Photosynthetic pigment transformations and decay constants, Estuaries 14, 65–73.
Boudreau B. P. (1996) Diagenetic Models and Their Implementation, Springer-Verlag.
Brendel, P. J. (1995) Development of a mercury thin film voltammetric microelectrode for the determination of biogeochemically important redox species in porewaters of marine and freshwater sediments, Ph.D., University of Delaware.
Brendel, P. J. and Luther, G. W., III. (1995) Development of a gold amalgam voltammetric microelectrode for the determination of dissolved Fe, Mn, O2, and S(-II) in porewaters of marine and freshwater sediments, Environmental Science & Technology 29, 751–761.
Burdige, D. J. (1991) The kinetics of organic matter mineralization in anoxic marine sediments, Journal of Marine Research 49, 727–761.
Burdige, D. J. and Zimmerman, R. C. (2002) Impact of sea grass density on carbonate dissolution in Bahamian sediments, Limnology and Oceanography 47(6), 1751–1763.
Caçador, I., Vale, C., and Catarino, F. (2000) Seasonal variation of Zn, Pb, Cu and Cd concentrations in the root-sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Marine Environmental Research 49, 279–290.
Caetano, M. and Vale, C. (2002) Retention of arsenic and phosphorus in iron-rich concretions of Tagus salt marshes, Marine Chemistry 79, 261–271.
Catarino, F. and Caçador, I. (1981) Produçao de biomassa estragégia de desenvolvimento em Spartina maritima e outros elementos da vegetaçao dos sapais do estuario do Tejo, Boletim da Sociedade Broteriana 2, 384–403.
Christensen, K. K., Jensen, H. S., Andersen, F. Ø., Wigand, C., and Holmer, M. (1998) Interferences between root plaque formation and phosphorus availability for isoetids in sediments of oligotrophic lakes, Biogeochemistry 43, 107–128.
Clothier, B. R. and Green, S. R. (1997) Roots: The big movers of water and chemicals in soil. Soil Science 162(8), 534–543.
Conlin, T. S. S. and Crowder, A. A. (1989) Location of radial oxygen loss and zones of potential iron uptake in a grass and two nongrass emergent species, Can. J. Bot. 67, 717–722.
D'Andrea, A. F., Aller, R. C., and Lopez, G. R. (2002) Organic matter flux and reactivity on a South Carolina sandflat: The impacts of porewater advection and macrobiological structures, Limnology & Oceanography 47(2), 1056–1070.
Giblin, A. E. and Howarth, R. W. (1984) Porewater evidence for a dynamic sedimentary iron cycle in salt marshes, Limnology & Oceanography 29, 47–63.
Kostka, J. E., Gribsholt, B., Petrie, E., Dalton, D., Skelton, H., and Kristensen, E. (2002) The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments, Limnology & Oceanography 47(1), 230–240.
Kostka, J. E. and Luther, G. W., III (1995) Seasonal cycling of Fe in saltmarsh sediments, Biogeochemistry 29, 159–181.
Krom, M. D. and Berner, R. A. (1980) Adsorption of phosphate in anoxic marine sediments, Limnology & Oceanography 25, 797–806.
Krom, M. D. and Berner, R. D. (1981) The diagenesis of phosphorus in a nearshore marine sediment, Geochimica et Cosmochimica Acta 45, 207–216.
Luther G. W., III and Church T. M. (1988) Seasonal cycling of sulfur and iron in porewaters of a Delaware saltmarsh, Marine Chemistry 23, 295–309.
Luther, G. W., III, Ferdelman, T. G., Kostka, J. E., Tsamakis, E. J., and Church, T. M. (1991) Temporal and spatial variability of reduced sulfur species (FeS2, S2O 2-3 ) and porewater parameters in salt marsh sediments, Biogeochemistry 14, 57–88.
Madureira, M. J., Vale C., and Gonçalves, M. L. (1997) Effect of plants on sulphur geochemistry in the Tagus salt-marshes sediments, Marine Chemistry 58, 27–37.
Meites, L. (1965) Polarographic Techniques, 2nd edn, Wiley, New York.
Rozan, T. F., Taillefert, M., Trouwborst, R. E., Glazer, B. T., Ma, S., Herszage, J. L., Valdes, M. K., Price, S., and Luther, I., G. W. (2002) Iron, sulfur and phosphorus cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms, Limnology & Oceanography 47, 1346–1354.
Schubauer, J. P. and Hopkinson, C. S. (1984) Above-and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia, Limnology & Oceanography 29(5), 1052–1065.
Stumm, W. and Morgan, J. J. (1996) Aquatic Chemistry, Wiley.
Sundby, B., Gobeil, C., Silverberg, N., and Mucci, N. (1992) The phosphorus cycle in coastal marine sediments, Limnology & Oceanography 37(6), 1129–1145.
Sundby, B., Vale, C., Caçador, I., Catarino, F., Madureira, M.-J., and Caetano, M. (1998) Metal-rich concretions on the roots of salt-marsh plants: Mechanism and rate of formation, Limnology & Oceanography 43, 19–26.
Sung, W. and Morgan, J. J. (1980) Kinetics and product of ferrous iron oxygenation in aqueous systems, Environmental Science and Technology 14, 561.
Taillefert, M. and Rozan, T. F. (2002) Electrochemical methods for the environmental analysis of trace elements biogeochemistry. In: Environmental Electrochemistry (eds. M. Taillefert and T. F. Rozan), American Chemical Society, pp. 2–13.
Teal, J. M. and Kanwisher, J. W. (1966) Gas transport in the marsh grass, Spartina alterniflora, J. Exp. Bot. 17, 355–361.
Vale, C., Catarino, F., Cortesão, C., and Caçador, I. (1990) Presence of metal-rich rhizoconcretions on he roots of Spartina maritima from the salt marshes of the Tagus estuary, Portugal, Science of the Total Environment 97/98, 617–626.
Westrich, J. T. and Berner, R. A. (1984) The role of sedimentary organic matter in bacterial sulfate reduction: The G model tested, Limnology & Oceanography 29, 236–249.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sundby, B., Vale, C., Caetano, M. et al. Redox Chemistry in the Root Zone of a Salt Marsh Sediment in the Tagus Estuary, Portugal. Aquatic Geochemistry 9, 257–271 (2003). https://doi.org/10.1023/B:AQUA.0000022957.42522.9a
Issue Date:
DOI: https://doi.org/10.1023/B:AQUA.0000022957.42522.9a