Abstract
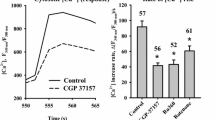
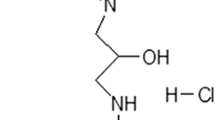
The excitotoxicity of glutamate is believed to be mediated by sustained increase in the cytosolic Ca2+ concentration. Mitochondria play a vital role in buffering the cytosolic calcium overload in stimulated neurons. Here we have studied the glutamate induced Ca2+ signals in cortical brain slices under physiological conditions and the conditions that modify the mitochondrial functions. Exposure of slices to glutamate caused a rapid increase in [Ca2+]i followed by a slow and persistently rising phase. The rapid increase in [Ca2+]i was mainly due to influx of Ca2+ through the N-methyl-D-aspartate (NMDA) receptor channels. Glutamate stimulation in the absence of Ca2+ in the extracellular medium elicited a small transient rise in [Ca2+]i which can be attributed to the mobilization of Ca2+ from IP3 sensitive endoplasmic reticulum pools consequent to activation of metabotropic glutamate receptors. The glutamate induced Ca2+ influx was accompanied by depolarization of the mitochondrial membrane, which was inhibited by ruthenium red, the blocker of mitochondrial Ca2+ uniporter. These results imply that mitochondria sequester the Ca2+ loaded into the cytosol by glutamate stimulation. Persistent depolarization of mitochondrial membrane observed in presence of extracellular Ca2+ caused permeability transition and released the sequestered Ca2+ which is manifested as slow rise in [Ca2+]i. Protonophore carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) depolarized the mitochondrial membrane and enhanced the glutamate induced [Ca2+]i response. Contrary to this, treatment of slices with mitochondrial inhibitor oligomycin or ruthenium red markedly reduced the [Ca2+]i response. Combined treatment with oligomycin and rotenone further diminished the [Ca2+]i response and also abolished the CCCP mediated rise in [Ca2+]i. However, rotenone alone had no effect on glutamate induced [Ca2+]i response. These changes in glutamate-induced [Ca2+]i response could not be explained on the basis of deficient mitochondrial Ca2+ sequestration or ATP dependent Ca2+ buffering. The mitochondrial inhibitors reduced the cellular ATP/ADP ratio, however, this would have restrained the ATP dependent Ca2+ buffering processes leading to elevation of [Ca2+]i. In contrast our results showed repression of Ca2+ signal except in case of CCCP which drastically reduced the ATP/ADP ratio. It was inferred that, under the conditions that hamper the Ca2+ sequestering ability of mitochondria, the glutamate induced Ca2+ influx could be impeded. To validate this, influx of Mn2+ through ionotropic glutamate receptor channel was monitored by measuring the quenching of Fura-2 fluorescence. Treatment of slices with oligomycin and rotenone prior to glutamate exposure conspicuously reduced the rate of glutamate induced fluorescence quenching as compared to untreated slices. Thus our data establish that the functional status of mitochondria can modify the activity of ionotropic glutamate receptor and suggest that blockade of mitochondrial Ca2+ sequestration may desensitize the NMDA receptor operated channel.
Similar content being viewed by others
REFERENCES
Benveniste, H., Drejer, J., Schousboe, A., and Diemer, N. H. 1984. Elevation of the extracellular concentration of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43:1369–1374.
Bullock, R., Butcher, S., Chen, S. P., Kendall, M. H., and Mc-Culloch, J. 1991. Correlation of the extracellular glutamate concentration with extent of blood flow reduction after subdural hematoma in the rat. J. Neurosurg. 94:794–802.
Meldrum, B. and Garthwaite, J. 1990. Excitatory aminoacid neurotoxicity and neurodegenerative diseases. Trends Pharmacol. Sci. 11:54–62.
Choi, D. W. 1987. Ionic dependence of glutamate excitotoxicity. J. Neurosci. 7:369–379.
Dubinsky, J. M. 1993. Intracellular calcium during the period of delayed excitotoxicity. J. Neurosci. 13:623–631.
Olney, J. W. 1969. Brain lesions, obesity and other disturbances in mice treated with monosodium glutamate. Science 164:719–721.
Eimerl, S. and Schramm, M. 1994. The quantity of calcium that appears to induce neuronal death. J. Neurochem. 62:1223–1226.
Hartley, D. M., Kurth, M. C., Bjerkness, L., Weiss, J. M., and Choi, D. W. 1993. Glutamate receptor induced 45Ca2+accumulation in cortical cell cultures correlates with subsequent neuronal degeneration. J. Neurosci. 13:1993–2000.
Khodorov, B. I., Pinelis, V. G., Vergun, O., Storozhevykh, T., and Vinskya, N. 1993. On the origin of a sustained increase in cytosolic calcium concentration after toxic glutamate treatment of the nerve cell culture. FEBS. Lett. 324:271–273.
Tymianski, M., Charlton, M., Carlen, P., and Tator, C. 1993. Secondary Ca2+overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 607: 319–323.
Schinder, A. F., Olson, E. C., Spitzer, N. C., and Montal, M. 1996. Mitochondrial dysfunction is a primary event in glutamate excitotoxicity. J. Neurosci. 16:6125–6133.
Budd, S. L. and Nicholls, D. G. 1996. Mitochondria, calcium regulation and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem. 67:2282–2291.
Khodorov, B. I., Pinelis, V. G., Vergun, O., Storozhevykh, T., and Vinskya, N. 1996. Dominant role of mitochondria in protection against a delayed neuronal Ca2+ overload induced by endogenous excitatory amino acids following a glutamate pulse. FEBS Lett. 393:135–136.
Khodorov, B., Pinelis, V. G., Storozhevykh, T., Yuravichus, A. and Khaspekhov, L. 1999. Blockade of mitochondrial Ca2+uptake by mitochondrial inhibitors amplifies the glutamate induced calcium response in cultured cerebellar granule cells. FEBS Lett. 458:162–166.
White, R. J. and Reynolds, I. J. 1997. Mitochondria accumulate calcium following intense glutamate stimulation of cultured rat forebrain neurons. J. Physiol. (Lond.) 498:31–47.
Castilho, R. F., Hansson, O., Ward, M. W., Budd, S. L., and Nicholls, D. G. 1998. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 18:10277–10286.
White, R. J. and Reynolds, I. J. 1996. Mitochondrial depolarization in glutamate stimulated neurons: An early signal specific to excitotoxic exposure. J. Neurosci. 16:5688–5697.
Ravichandra, B. and Joshi, P. G. 1999. Regulation of transmembrane signalling by ganglioside GM1: Interaction of anti-GM1 with neuro-2a cells. J. Neurochem. 73:557–567.
Grynkiewicz, G., Poenie, M., and Tsien, R. Y. 1985. A new generation of Ca2+indicators with greatly improved fluorescent properties. J. Biol. Chem. 260:3440–3450.
Bickler, P. E. and Gallego, S. M. 1993. Inhibition of brain calcium channels by plasma proteins from anoxic turtles. Am. J. Physiol. 265:R277–R281.
Kannurpatti, S. S. and Joshi, N. B. 1999. Energy metabolism and NAD-NADH redox state in brain slices in response to glutamate exposure and ischemia. Metab. Brain Dis. 14:33–43.
Khodorov, B. I., Pinelis, V. G., Vergun, O., Storozhevykh, T., and Vinskya, N. 1996. Mitochondrial deenergization underlies neuronal calcium overload following a prolonged glutamate challenge. FEBS Lett. 379:230–234.
Lohnson, L. V., Walsh, M. L., and Chen, L. B. 1980. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA. 77:990–994.
McKenna, M. C., Sonnewald, U., Haung, X., Stevenson, J. and Zielke, H. R. 1996. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J. Neurochem. 66:386–393.
Budd, S. L. and Nicholls, D. G. 1996. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 66:403–411.
Peng, T. I., Jou, M. J., Sheu, S. S., and Greenamyre, T. 1998. Visualization of NMDA receptor-induced mitochondrial calcium accumulation in striatal neurons. Exp. Neurol. 149:1–12.
Beatrice, M. C., Palmer, J. W., and Pfeiffer, D. R. 1980. The relationship between mitochondrial membrane permeability, membrane potential and the retention of Ca2+by mitochondria. J. Biol. Chem. 225:8663–8671.
Kristal, B. S. and Dubinsky, J. M. 1997. Mitochondrial permeability transition in the central nervous system: Induction by calcium cycling dependent and independent pathways. J. Neurochem. 69:524–538.
Constantini, P., Chernyak, B. V., Petronilli, V., and Bernardi, P. 1996. Modulation of the mitochondrial permeability transition by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 271:6746–6751.
Legendre, P., Rosemund, C., and Westbrook, G. L. 1993. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J. Neurosci. 13: 674–684.
Lieberman, D. N. and Mody, I. 1994. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature 369:235–239.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kannurpatti, S.S., Joshi, P.G. & Joshi, N.B. Calcium Sequestering Ability of Mitochondria Modulates Influx of Calcium through Glutamate Receptor Channel. Neurochem Res 25, 1527–1536 (2000). https://doi.org/10.1023/A:1026602100160
Issue Date:
DOI: https://doi.org/10.1023/A:1026602100160