Abstract
Purpose. To evaluate covariate effects on the pharmacokinetics of temozolomide in cancer patients, and to explore the dose-pharmacokinetics-toxicity relationship of temozolomide.
Methods. Non-linear mixed-effects modeling approach was used to analyze the data from 445 patients enrolled in eleven Phase I and Phase II clinical trials. All patients in the phase I trials had advanced cancer. Patients in the phase II trials had anaplastic astrocytoma (AA), glioblastoma multiforme (GBM) or malignant melanoma (MM). A sparse sampling scheme was prospectively developed using Phase I data and was successfully implemented in Phase II trials. Population factors included age, gender, height (HT), weight (WT), body surface area (BSA), serum creatinine (Sr.Cr.), estimated creatinine clearance, serum chemistry data as indices of hepatic function and disease, smoking status, and selected concomitant medications. Descriptive statistics were used to summarize the toxicity and temozolomide dose and exposure relationship.
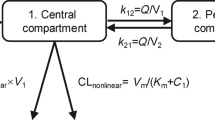
Results. The pharmacokinetics of temozolomide follows a one-compartment model with first order absorption and elimination. Temozolomide clearance (CL) increased with BSA for both genders. The population mean clearance for GBM or AA patients was 11.2 L/hr for male with BSA equal to 2.0 m2, and 8.8 L/hr for female with BSA equal to 1.7 m2. The mean clearance for MM patients was slightly higher. The inter-subject variability in clearance was 15%, and the residual variability was 26%. Other factors investigated in this analysis had little effect on clearance. The overall incidence of neutropenia and thrombocytopenia were 5-8%. Temozolomide dose and AUC did not predict nadir neutrophil and platelet counts due to large variability in counts.
Conclusions. The current dose regimen is administered according to BSA which is the most important factor influencing temozolomide clearance. No further dose adjustment is required.
Similar content being viewed by others
REFERENCES
M. F. Stevens, J. A. Hickman, R. Stone, N. W. Gibson, G. U. Baig, E. Lunt, and C. G. Newton. Antitumor imidazotetrazines I. Synthesis and chemistry of 8-carbamoyl-3(2-chloroethyl) imidazo [5,1,d]-1,2,3,5-tetrazin-4(3H)-one, a novel broad spectrum antitumor agent. J. Med. Chem. 27:196–201 (1984).
M. F. Stevens, J. A. Hickman, S. P. Langdon, et. al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-(2-chloroethyl) imidazo [5,1,d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M&B 39831) a novel drug with potential as an alternative to dacarbazine. Cancer Res. 47:5846–5852 (1987).
L. L. Tsang, C. P. Quarterman, A. Gescher, and J. A. Slack. Comparison of cytotoxicity in vitro of temozolomide and dacarbazine, prodrugs of 3-methyl-(triazen-1-yl) imidazole-4-carboxide. Cancer Chemother. Pharmacol. 27:342–346 (1991).
V. L. Bull and M. J. Tinsdale. Antitumor imidazotetrazines XVI. Macromolecular alkylation by 3-substituted imidazotetrazines. Biochem. Pharmacol. 36:3215–3220 (1987).
M. J. Tinsdale. Antitumor imidazotetrazines-XVIII. Modification of the level of 5-methylcytosine in DNA y 3-substituted imidazotetrazinones. Biochem. Pharmacol. 38:1097–1101 (1989).
L. L. Tsang, P. B. Farmer, A. Gescher, and J. A. Slack. Characterization of urinary metabolites of temozolomide in humans and mice and evaluation of their cytotoxicity. Cancer Chemother. Pharmacol. 26:429–436 (1990).
A. S. Clark, M. F. Stevens, C. E. Sansom, and C. H. Schwalbe. Antitumor imidazotetrazines. XXI. Mitozolamide and temozolomide: probes for the major groove of DNA. Anticancer Drug Design 5:63–68 (1990).
J. D. Minna, G. A. Higgins, and E. J. Glapstein. Cancer of the lung: In V. Devita, S. Hellman, S. Rosenberg (eds.), Cancer: Principles and Practice of Oncology, Lippincott, Philadelphia, 1984, p. 536.
D. DuBois and E. F. DuBois. A formula to estimate the approximate surface area if height and weight are known. Arch. Intern. Med. 17:863–867 (1976).
E. S. Newland, G. R. Blackledge, J. A. Slack, et. al. Phase I Trial of Temozolomide. Br. J. Cancer. 65(2):287–91 (1992).
D. W. Cockcroft and M. H. Gault. Prediction of creatinine clearance from serum creatinine. Nephron. 16:31–41 (1976).
L. B. Sheiner and S. L. Beal. Evaluation of methods for estimating population pharmacokinetic parameters: II. Bioexponential model; experimental pharmacokinetic Data. J. Pharmacokinet. Biopharm. 9:635–651 (1981).
L. B. Sheiner and S. L. Beal. Evaluation of methods for estimating population pharmacokinetic parameters: III. Monoexponential model; routine clinical pharmacokinetic data. J. Pharmacokinet. Biopharm. 11:303–319 (1983).
S. L. Beal and L. B. Sheiner. NONMEM Users Guides. NONMEM Project Group, UCSF, 1992.
SAS/STAT User's Guide. Version 6, SAS Institute, Inc., Cary, NC, 1990.
S-PLUS User's Manual. Version 3.2, MathSoft, Inc., Seattle, WA, 1993.
J. A. Slack, C. Golddard, M. F. G. Stevens, et. al. The analysis and murine pharmacokinetics of a new antitumor agent: CCRG 81045. J. Pharm. Pharmacol. 38:63P (1986).
R. T. Wheelhouse and M. F. G. Stevens. Decomposition of the antitumor drug temozolomide in deuteriated phosphate buffer: methyl group transfer is a accompanied by deuterium exchange. J. Chem. Soc. Chem. Commun. 15:1177–1178 (1993).
B. J. Denny, R. T. Wheelhouse, M. F. Stevens, L. L. Tsang, and J. A. Slack. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 33:9045–9051 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jen, J.F., Cutler, D.L., Pai, S.M. et al. Population Pharmacokinetics of Temozolomide in Cancer Patients. Pharm Res 17, 1284–1289 (2000). https://doi.org/10.1023/A:1026403805756
Issue Date:
DOI: https://doi.org/10.1023/A:1026403805756