Abstract
Purpose. Two different monoclonal antibody-targeted HPMA copolymer-doxorubicin conjugates, classic and starlike, were synthesized to be used for site-specific cancer therapy. The anti-mouse Thy-1.2 (IgG3) and two anti-human CD71/A (IgG1) and CD71/B (IgG2a) monoclonal antibodies were used as targeting structures.
Methods. Their binding and cytotoxic activity in vitro, body distribution, and anticancer activity in vivo were evaluated.
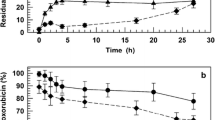
Results. The results of flow cytometric analysis showed comparable binding of classic and starlike conjugates to the target cells. The in vitro cytotoxic effect was 10-fold higher if cancer cells were exposed to the starlike conjugate compared to the classic one. Biodistribution studies showed that the starlike conjugate remained in a relatively high concentration in blood, whereas the classic conjugate was found in a 6.5-times lower amount. In contrast to the low antitumor activity of free doxorubicin and nontargeted HPMA copolymer-doxorubicin conjugate, both anti-Thy-1.2 targeted conjugates (classic and starlike) cured all mice bearing T-cell lymphoma EL4. On the other hand, starlike conjugates containing anti-CD71/A or anti-CD71/B monoclonals as targeting structures were more effective against human colorectal cancer SW 620 than the classic one.
Conclusions. We have shown that the starlike conjugates are more effective systems for targeted drug delivery and cancer treatment than classic conjugates.
Similar content being viewed by others
References
B. Říhová, J. Strohalm, K. Kubáčková, M. Jelínková, L. Rozprimová, M. Šírová, D. Plocová, T. Mrkvan, M. Kovár, J. Pokorná, T. Etrych, and K. Ulbrich. Drug-HPMA-HuIg conjugates effective against human solid cancer. Adv. Exp. Med. Biol. 519:125-143 (2003).
J. Kopeček, P. Rejmanová, J. Strohalm, K. Ulbrich, B. Říhová, V. Chytrý, J. B. Lloyd, and R. Duncan. Synthetic polymeric drugs. U.S. Patent. 5,037,883 (1991).
B. Říhová, P. Kopečková, J. Strohalm, P. Rossmann, V. Větvička, and J. Kopeček. Antibody-directed affinity therapy applied to the immune system: in vivo effectiveness and limited toxicity of daunomycin conjugated to HPMA copolymers and targeting antibody. Clin. Immunol. Immunopathol. 46:100-114 (1988).
B. Říhová, M. Jelínková, J. Strohalm, V. Šubr, D. Plocová, O. Hovorka, M. Novák, D. Plundrová, Y. Germano, and K. Ulbrich. Polymeric drugs based on conjugates of synthetic and natural macromolecules. II. Anti-cancer activity of antibody or (Fab')(2)-targeted conjugates and combined therapy with immunomodulators. J. Control. Release 64:241-261 (2000).
M. Kovář, J. Strohalm, K. Ulbrich, and B. Říhová. In vitro and in vivo effect of HPMA copolymer-bound doxorubicin targeted to transferrin receptor of B-cell lymphoma 38C13. J. Drug Target. 10:23-30 (2002).
M. Kovář, J. Strohalm, T. Etrych, K. Ulbrich, and B. Říhová. Star structure of antibody-targeted HPMA copolymer-bound doxorubicin: a novel type of polymeric conjugate for targeted drug delivery with potent antitumor effect. Bioconjug. Chem. 13:206-215 (2002).
B. Říhová, J. Strohalm, K. Kubáčková, M. Jelínková, O. Hovorka, M. Kovář, D. Plocová, M. Šírová, M. Št'astný, L. Rozprimová, and K. Ulbrich. Acquired and specific immunological mechanisms co-responsible for efficacy of polymer-bound drugs. J. Control. Release 78:97-114 (2002).
K. Ulbrich, M. Pechar, J. Strohalm, V. Šubr, and B. Říhová. Polymeric carriers of drugs for site-specific therapy. Macromol Symp. 118:577-585 (1997).
K. Ulbrich, V. Šubr, J. Strohalm, D. Plocová, M. Jelínková, and B. Říhová. Polymeric drugs based on conjugates of synthetic and natural macromolecules. I. Synthesis and physico-chemical characterisation. J. Control. Release 64:63-79 (2000).
K. Ulbrich, M. Pechar, J. Strohalm, V. Šubr, and B. Říhová. Synthesis of biodegradable polymers for controlled drug release. Ann. N. Y. Acad. Sci. 831:47-56 (1997).
K. Ulbrich, V. Šubr, M. Pechar, J. Strohalm, M. Jelínková, and B. Říhová. Hydrophilic polymers for drug delivery. Macromol. Symp. 152:151-162 (2000).
T. Ghose, A. Guclu, J. Tai, M. Mammen, and S. T. Norvell. Immunoprophylaxis and immunotherapy of EL4 lymphoma. Eur. J. Cancer 13:925-935 (1977).
T. Etrych, P. Chytil, M. Jelínková, B. Říhová, and K. Ulbrich. Synthesis of HPMA copolymers containing doxorubicin bound via a hydrazone linkage. Effect of spacer on drug release and in vitro cytotoxicity. Macromol. Biosci. 2:43-52 (2002).
V. Omelyanenko, P. Kopečková, C. Gentry, J. G. Shiah, and J. Kopeček. HPMA copolymer-anticancer drug-OV-TL16 antibody conjugates. 1. influence of the method of synthesis on the binding affinity to OVCAR-3 ovarian carcinoma cells in vitro. J. Drug Target. 3:357-373 (1996).
B. Říhová, A. Jegorov, and J. Strohalm. V. Mat'ha, P. Rossmann, L. Fornůsek, and K. Ulbrich. Antibody-targeted cyclosporin A. J. Control. Release 19:25-39 (1992).
B. Říhová and N. L. Krinick. and J. Kopeček. Targetable photoactivatable drugs. 3. Specific in vitro destruction of a human hepatocarcinoma cell line (PLC/PRF/5) or mouse splenocytes by polymer bound chlorin e6, targeted by galactosamine or anti-thy-1.2 antibodies, respectively. J. Control. Release 25:71-87 (1993).
Z. R. Lu, P. Kopečková, and J. Kopeček. Polymerizable Fab' antibody fragments for targeting of anticancer drugs. Nature Biotechnol. 17:1101-1104 (1999).
B. Říhová. Receptor-mediated targeted drug or toxin delivery. Adv. Drug Deliv. Rev. 29:273-289 (1998).
O. W. Press. P. J. Martin, P. E. Thorpe, and E. S. Vitetta. Ricin A-chain containing immunotoxins directed against different epitopes on the CD2 molecule differ in their ability to kill normal and malignant T cells. J. Immunol. 141:4410-4417 (1988).
B. Říhová, K. Vereš, L. Fornůsek, K. Ulbrich, J. Strohalm, V. Větvička, M. Bilej, and J. Kopeček. Action of polymeric prodrugs based on N-(2-hydroxypropyl)-methacrylamide copolymers. II. Body distribution and T-cell accumulation of free and polymer-bound [125I]daunomycin. J. Control. Release 10:37-49 (1989).
L. W. Seymour, P. A. Flanagan, A. Al-Shamkhani, V. Šubr, K. Ulbrich, J. Cassidy, and R. Duncan. Biological properties of monoclonal antibodies conjugated to synthetic polymeric drug carriers. Select Cancer Ther. 7:59-73 (1991).
P. A. Flanagan, V. Šubr, K. Ulbrich, P. Kopečková, J. Kopeček, and R. Duncan. Evaluation of protein-N-(2-hydroxypropyl) methacrylamide copolymer conjugates as targetable drug-carriers. 2. Body distribution of conjugates containing transferrin, antitransferrin receptor antibody or anti-Thy 1.2 and effectiveness of transferrin-containing daunomycin conjugates against mouse L1210 leukaemia in vivo. J. Control. Release 18:25-38 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jelínková, M., Strohalm, J., Etrych, T. et al. Starlike vs. Classic Macromolecular Prodrugs: Two Different Antibody-Targeted HPMA Copolymers of Doxorubicin Studied in Vitro and in Vivo as Potential Anticancer Drugs. Pharm Res 20, 1558–1564 (2003). https://doi.org/10.1023/A:1026170830782
Issue Date:
DOI: https://doi.org/10.1023/A:1026170830782