Abstract
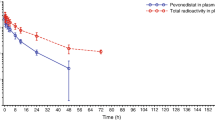
The aim of this multicenter study was to determine whether valspodar (Amdray™; code designation, SDZ PSC 833), a potent P-glycoprotein (P-gp) inhibitor, affects the pharmacokinetics of unbound paclitaxel (C u). Data were obtained from 31 patients with advanced breast cancer. Thirteen patients were treated with paclitaxel alone (3-h infusion at 175mg/m2) and another 18 received paclitaxel (3-h infusion at 70mg/m2) in combination with a 21-day cycle of oral valspodar (5mg/kg given four times a day) starting 1 day before administration of paclitaxel. Serial blood samples were taken in the first course and C u in plasma determined using equilibrium dialysis with a [G-3H]paclitaxel tracer. The apparent clearance of C u was not significantly different between the two groups, with mean ± standard deviation (±SD) values of 230 ± 56.0 and 202 ± 49.9L/h/m2 in the absence and presence of valspodar, respectively (P = 0.17). The volume of C u distribution was slightly larger in the presence of valspodar (1160 ± 474 vs. 1620 ± 552L/m2; P = 0.025), which contributed to a minor difference in the terminal disposition half-life (6.12 ± 3.42 vs. 8.50 ± 2.06h; P = 0.028). These data indicate that (i) valspodar lacks the significant interaction with paclitaxel observed previously with other P-gp modulators, (ii) the majority of the increased toxicity of the combination does not appear to be attributable to increased levels of C u, and (iii) provide further evidence of the conjecture that the plasma concentration of paclitaxel may not be an appropriate measure to monitor the impact of P-gp inhibition.
Similar content being viewed by others
References
Gottesman MM, Fojo T, Bates S: Multidrug resistance in cancer: role of AT-dependent transporters. Nature Rev Cancer 2: 48–58, 2002
Van Zuylen L, Nooter K, Sparreboom A, Verweij J: Development of multidrug-resistance convertors: sense or nonsense? Invest New Drugs 18: 205–220, 2000
Sparreboom A, Nooter K: Does P-glycoprotein play a role in anticancer drug pharmacokinetics? Drug Resis Updates 3: 357–363, 2000
Kroemer HK, Gautier JC, Beaune P, Henderson C, Wolf CR, Eichelbaum M: Identification of P450 enzymes involved in metabolism of verapamil in humans. Arch Pharmacol 348: 332–337, 1993
Kronbach T, Fischer V, Meyer UA: Cyclosporine metabolism in human liver: identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther 43: 630–635, 1988
Fischer V, Rodriguez-Gaston A, Heitz F, Tynes R, Hauck C, Cohen D, Vickers AE: The multidrug resistance modulator valspodar (PSC 833) is metabolized by human cytochrome P450 3A: implications for drug-drug interactions and pharmacological activity of the main metabolite. Drug Metab Dispos 26: 802–811, 1998
Boesch D, Gaveriaux C, Jachezs B, Poutier-Manzanedo A, Bollinger P, Loor F: In vivo circumvention of P-glycoprotein mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res 51: 4226–4233, 1991
Boote D, Dennis P, Twentyman P, Osborne RJ, Hensel S, Smyth JF, Brampton MH, Bleehen NM: Phase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancer. J Clin Oncol 14: 610–618, 1996
Giaccone G, Linn SC, Welink J, Catimel G, Stieltjes H, Van der Vijgh WJ, Eeltink C, Vermorken JB, Pinedo HM: A dose finding and pharmacokinetic study of reversal of multidrug resistance with PSC 833 in combination with doxorubicin in patients with solid tumors. Clin Cancer Res 3: 2005–2015, 1997
Harris JW, Rahman A, Kim BR, Guengerich FP, Collins JM: Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res 54: 4026–4035, 1994
Arzoo KK, Doroshow J, Frankel P, Gandara D, Groshen S, Muggia F, Russell C, Spicer DC, Synold T, Waisman J, Warner E: Valspodar (V) + paclitaxel (P) in advanced breast cancer: a California Cancer Consortium phase II randomized trial. Proc Am Soc Clin Oncol 19: 205a, 2000 (Abstract)
Brouwer E, Verweij J, De Bruijn P, Loos WJ, Pillay M, Buijs D, Sparreboom A: Measurement of fraction unbound paclitaxel in human plasma. Drug Metab Dispos 28: 1141–1145, 2000
Sparreboom A, De Bruijn P, Nooter K, Loos WJ, Stoter G, Verweij J: Determination of paclitaxel in human plasma using single solvent extraction prior to isocratic reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B 705: 159–164, 1998
Sparreboom A, Loos WJ, Verweij J, De Vos AI, Van der Burg MEL, Stoter G, Nooter K: Quantitation of Cremophor EL in human plasma samples using a colorimetric dye-binding microassay. Anal Biochem 255: 171–175, 1998
Brouwer E, Verweij J, Hauns B, Loos WJ, Nooter K, Mross K, Stoter G, Sparreboom A: Linearized colorimetric assay for Cremophor EL. application to pharmacokinetics after 1–hour paclitaxel infusions. Anal Biochem 261: 198–202, 1998
Henningsson A, Karlsson MO, Vigano L, Gianni L, Verweij J, Sparreboom A: Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol 19: 4065–4073, 2001
Sparreboom A, Verweij J, Van der Burg MEL, Loos WJ, Brouwer E, Vigano L, Locatelli A, De Vos, AI, Nooter K, Stoter G, Gianni L: Disposition of Cremophor EL in humans limits the potential for modulation of the multidrug resistance phenotype. Clin Cancer Res 4: 1937–1942, 1998
Gelderblom H, Mross K, Ten Tije AJ, Behringer D, Mielke S, Van Zomeren DM, Verweij J, Sparreboom A: Comparative pharmacokinetics of unbound paclitaxel during 1–and 3–hour infusions. J Clin Oncol 20: 574–581, 2002
Smorenburg CH, Ten Tije AJ, Verweij J, Bontenbal M, Mross K, Van Zomeren DM, Seynaeve C, Sparreboom A: Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer 39: 196–202, 2003
Van Zuylen L, Gianni L, Verweij J, Mross K, Brouwer E, Loos WJ, Sparreboom A: Interrelationships of paclitaxel disposition, infusion duration and Cremophor EL kinetics in cancer patients. Anticancer Drugs 11: 3331–3337, 2000
Collins HL, Fisher GA, Hausdorff J, Lum BL, Pearce T, Halsey J, Sikic BI: Phase I study of paclitaxel in combination with SDZ PSC 833, a multidrug resistance modulator. Proc Am Soc Clin Oncol 14: 181a, 1995 (Abstract)
Fracasso PM, Westerveldt P, Fears CA, Rosen M, Zuhowski EG, Cazenave LA, Litchman M, Egorin MJ: Phase I study of paclitaxel in combination with a multidrug resistance modulator, PSC 833 (valspodar), in refractory malignancies. J Clin Oncol 18: 1124–1134, 2000
Patnaik A, Warner E, Michael M, Egorin MJ, Moore MJ, Siu LL, Fracasso PM, Rivkin S, Kerr I, Litchman M, Oza AM: Phase I dose-finding and pharmacokinetic study of paclitaxel and carboplatin with oral valspodar in patients with advanced solid tumors. J Clin Oncol 18: 3677–3689, 2000
Advani R, Fisher GA, Lum BL, Hausdorff J, Halsey J, Litchman M, Sikic BI: A phase I trial of doxorubicin, paclitaxel, and valspodar (PSC 833), a modulator of multidrug resistance. Clin Cancer Res 7: 1221–1229, 2001
Chico I, Kang MH, Bergan R, Abraham J, Bakke S, Meadows B, Rutt A, Robey R, Choyke P, Merino M, Goldspiel B, Smith T, Steinberg S, Figg WD, Fojo T, Bates S: Phase I study of infusional paclitaxel in combination with the P-glycoprotein antagonist PSC 833. J Clin Oncol 19: 832–842, 2001
Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Locatelli A, Bonadonna G, Egorin MJ: Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13: 180–190, 1995
Van Zuylen L, Karlsson MO, Verweij J, Brouwer E, De Bruijn P, Nooter K, Stoter G, Sparreboom A: Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol 47: 309–318, 2001
Gelderblom H, Verweij J, Nooter K, Sparreboom A: Cremophor EL: the downsides and potential of vehicle selection for drug formulation. Eur J Cancer 37: 1587–1595, 2001
Van Zuylen L, Verweij J, Sparreboom A: Role of formulation vehicles in taxane pharmacology. Invest New Drugs 19: 125–141, 2001
Sparreboom A, Van Zuylen L, Brouwer E, Loos WJ, De Bruijn P, Gelderblom H, Pillay M, Nooter K, Stoter G, Verweij J: Cremophor EL mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res 59: 1454–1457, 1999
Sparreboom A, Van Tellingen O, Nooijen WJ, Beijnen JH: Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res 56: 2112–2115, 1996
Sparreboom A, Van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DKF, Borst P, Nooijen WJ, Beijnen JH, Van Tellingen O: Limited oral bioavailability and active epithelial excretion of paclitaxel caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA 94: 2031–2035, 1997
Van Zuylen L, Sparreboom A, Van der Gaast A, Van der Burg MEL, Van Beurden V, Bol CJ, Woestenborghs R, Palmer PA, Verweij, J: The orally administered P-glycoprotein inhibitor R101933 does not alter the plasma pharmacokinetics of paclitaxel. Clin Cancer Res 6: 1365–1371, 2000
Van Zuylen L, Verweij J, Nooter K, Brouwer E, Stoter G, Sparreboom A: Role of intestinal P-glycoprotein in the plasma and fecal disposition of docetaxel in humans. Clin Cancer Res 6: 2598–2603, 2000
Kearns CM, Gianni L, Egorin MJ: Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 22: 16–23, 1995
Kang MH, Figg WD, Ando Y, Blagosklonny MV, Liewehr D, Fojo T, Bates SE: The P-glycoprotein antagonist PSC 833 increases the plasma concentrations of 6α-hydroxypaclitaxel, a major metabolite of paclitaxel. Clin Cancer Res 7: 1610–1617, 2001
Rahmani A, Korzekwa KR, Grohan J, Gonzalez FJ, Harris JW: Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res 54: 5543–5546, 1994
Karlsson MO, Molnar V, Bergh J, Freijs A, Larsson R: A general model for time-dissociated pharmacokinetic-pharmacodynamic relationship exemplified by paclitaxel myelosuppression. Clin Pharmacol Ther 63: 11–25, 1998
Minami H, Sasaki Y, Saijo N, Ohtsu T, Fujii H, Igarashi T, Itoh K: Indirect-response model for the time course of leukopenia with anticancer drugs. Clin Pharmacol Ther 64: 511–521, 1998
Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M: Subpopulations of normal blood and bone marrow cells express a functional multidrug resistance phenotype. Blood 80: 2729–2734, 1992
Tidefelt U, Liliemark J, Gruber A, Liliemark E, Sundman-Engberg B, Juliusson G, Stenke L, Elmhorn-Rosenborg A, Mollgard L, Lehman S, Xu D, Covelli A, Gustavsson B, Paul C: P-glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with P-glycoprotein-positive acute myeloid leukemia. J Clin Oncol 18: 1837–1844, 2000
Lum BL, Kaubisch S, Fisher GA, Brown BW, Sikic BI: Effect of high-dose cyclosporine on etoposide pharmacodynamics in a trial to reverse P-glycoprotein (MDR1 gene) mediated drug resistance. Cancer Chemother Pharmacol 45: 305–311, 2000
Jachez B, Nordmann R, Loor F: Restoration of taxol sensitivity of multidrug-resistance cell by the cyclosporine SDZ PSC 833 and the cyclopeptolide SDZ 280–446. J Natl Cancer Inst 85: 478–483, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ten Tije, A.J., Synold, T.W., Spicer, D. et al. Effect of valspodar on the pharmacokinetics of unbound paclitaxel. Invest New Drugs 21, 291–298 (2003). https://doi.org/10.1023/A:1025412509730
Issue Date:
DOI: https://doi.org/10.1023/A:1025412509730