Abstract
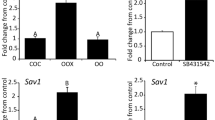
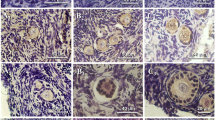
Ovulation is a complex, multi-factorial event that involves the degeneration of a specific area of the follicular and ovarian surface via apoptosis. Many apoptosis related genes have been identified in the ovary. Secreted Frizzled Related Protein 4 (sFRP4) is a protein that appears to antagonize a molecular pathway for cell survival. sFRP4 gene expression is known to be upregulated with apoptosis in the ovarian corpus luteum. In this study, ovulation was hormonally induced in immature Wistar rats and their ovaries collected for analysis of apoptosis and sFRP4. TUNEL staining identified a greater amount of dying cells in the thecal layer of treated rat ovaries compared to controls. The results of 3′-end labelling revealed a significant increase (p < 0.01) in apoptosis at 12 hours following treatment compared to other time points and control. In situ hybridization exhibited a visible increase in amounts of sFRP4 mRNA expression in the thecal layers of follicles from treated rats compared to controls. Quantitative RT-PCR revealed no significant difference in sFRP4 expression levels between treated and control tissues although a clear trend towards an increase was observed in the treated group. This study demonstrates an association between sFRP4 and apoptosis in rat ovulation.
Similar content being viewed by others
References
Epsey LL. Ovulation as an inflammatory reaction-a hypothesis. Biol Reprod 1980; 22: 73-106.
Ackerman RC, Murdoch WJ. Prostaglandin-induced apoptosis of ovarian surface epithelial cells. Prostaglandins 1993; 45: 475-485.
Hseuh AJW, Billig H, Tsafriri A. Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocr Rev 1994; 15: 707-724.
Bielke W, Guo K, Buhrer S, Saurer S, Friis RR. Apoptosis in the rat mammary gland and ventral prostate: Detection of cell death associated genes using a coincident expression cloning approach. Cell Death Differ 1997; 4: 114-124.
Guo K, Wolf V, Dharmarajan AM, et al. Apoptosis associated gene expression in the CL of the rat. Biol Reprod 1998; 58: 739-746.
Finch PW, He X, Kelley MJ, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA 1997; 94: 6770-6775.
Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 1999; 274(23): 16180-16187.
Wodarz A, Nusse R. Mechanisms of Wnt signalling in development. Anny Rev Cell Dev Biol 1998; 14: 59-88.
Dierick H, Bejsovec A. Cellular mechanisms of wingless/ Wnt signal transduction. Curr Top Dev Biol 1999; 43: 153-190.
Nusse R, Varmus HE. Wnt genes. Cell 1992; 69: 1073-1087.
Parr BA, McMahon AP. Wnt genes and vertebrate development. Curr Opin Genet Dev 1994; 4(4): 523-528.
Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 1998; 395(6703): 707-710.
Vaino S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999; 397: 405-409.
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31(1): 99-109.
Uren A, Reichsman F, Anest V, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 2000; 275(6): 4374-4382.
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998; 391(6665): 357-362.
Hsieh JC, Kodjabachian L, Rebbert ML, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 1999; 398(6726): 431-436.
Marti A, Jaggi R, Vallan C, et al. Physiological apoptosis in hormone-dependant tissues: Involvement of caspases. Cell Death Differ 1999; 6: 1190-1200.
Pesce MA, Bodourian SH, Sheehan M, Henkel CF. An automated biotin-streptavidin procedure for progesterone evaluation. Clin Biochem 1992; 25(6): 451-455.
Tilly JL, Hseuh AJ. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol 1993; 154: 519-526.
Waddell BJ, Hisheh S, Dharmarajan AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod 2000; 63: 1913-1917.
Roughton SA, Lareu RR, Bittles AH, Dharmarajan AM. Fas and Fas ligand messenger ribonucleic acid and protein expression in the rat corpus luteum during apoptosis-mediated luteolysis. Biol Reprod 1999; 60(4): 797-804.
Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol 1991; 5(10): 1405-1417.
Orly J, Rei Z, Greenberg NM, Richards JS. Tyrosine kinase inhibitor AG18 arrests follicle-stimulating hormone-induced granulosa cell differentiation: Use of reverse transcriptasepolymerase chain reaction assay for multiple messenger ribonucleic acids. Endocrinology 1994; 134(6): 2336-2346.
Talbot P, Martin GG, Ashby H. Formation of the rupture site in preovulatory hamster and mouse follicles: Loss of the surface epithelium. Gamete Research 1987; 17(4): 287-302.
Murdoch W. Ovarian surface epithelium during ovulatory and anovulatory ovine estrous cycles. Anat Rec 1994; 240: 3222-3326.
Niswender GD, Nett TM. The corpus luteum and its control in infraprimate species. In: Knobil E, Neill JD, eds. Physiology of Reproduction 2nd ed., NY: Raven Press, 1994; 781-816.
Piquette GN, Tilly JL, Prichard LE, Simon C, Polan ML. Detection of apoptosis in human and rat ovarian follicles. J Soc Gynecol Investig 1994; 1(4): 297-301.
Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, eds., The Physiology of Reproduction. New York: Raven Press, 1994; 725-780.
Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Fizzled homologue triggers G-protein-linked phophatidylinositol signalling. Nature 1997; 390: 410-413.
Espey LL. A review of factors that could influence membrane potentials of ovarian follicular cells during mammalian ovulation. Acta Endocrinol 1992; 126(2): 1-31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drake, J.M., Friis, R.R. & Dharmarajan, A.M. The role of sFRP4, a secreted frizzled-related protein, in ovulation. Apoptosis 8, 389–397 (2003). https://doi.org/10.1023/A:1024181203729
Issue Date:
DOI: https://doi.org/10.1023/A:1024181203729