Abstract
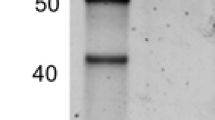
An α-L-fucosidase purified from pea (Pisum sativum L. cv Alaska) epicotyl was previously described as a cell wall enzyme of 20 kDa that hydrolyses terminal α-L-fucosidic linkages from oligosaccharide fragments of xyloglucan. cDNA and genomic copies were further isolated and sequenced. The predicted product of the cDNA and the genomic clone (fuc1), was a 20 kDa protein containing a signal peptide and five cysteines. This was the first α-L-fucosidase gene to be cloned in plants but its fucosidase activity has not been demonstrated. Here, our biochemical and immuno analyses suggest that fuc1 does not encode an α-L-fucosidase. Pea fuc1 expressed in Escherichia coli, insect cells and Arabidopsis thaliana produced recombinant proteins without α-L-fucosidase activity. Pea plants had endogenous α-L-fucosidase activity, but the enzyme was not recognised by an antibody produced against recombinant FUC1 protein expressed in E. coli. In contrast, the antibody immunoprecipitated a 20 kDa protein which was inactive. By chromatographic analysis of pea protein extracts, we separated α-L-fucosidase-active fractions from the 20 kDa protein fractions. We conclude that the α-L-fucosidase activity is not attributable to the 20 kDa FUC1 protein. A new function for fuc1 gene product, now named PIP20 (for protease inhibitor from pea) is proposed.
Similar content being viewed by others
References
Augur, C., Benhamou, N., Darvill, A. and Albersheim, P. 1993. Purification, characterization, and cell wall localization of an ?-fucosidase that inactivates a xyloglucan oligosaccharin. Plant J. 3: 415–426.
Augur, C., Stiefel, V., Darvill, A., Albersheim, P. and Puigdomenech, P. 1995. Molecular cloning and pattern of expression of an alpha-L-fucosidase gene from pea seedlings. J. Biol. Chem. 270: 24839–24843.
Bahl, O.P. 1970. Glycosidases of Aspergillus niger. II. Purification and general properties of 1,2-?-L-fucosidase. J. Biol. Chem. 245: 299–304.
Bevan, M. 1984. Binary Agrobacterium vectors for plant transformation. Nucl. Acids Res. 12: 8711–8721.
Blow, D.M., Janin, J. and Sweet, R.M. 1974. Mode of action of soybean trypsin inhibitor (Kunitz) as a model for specific proteinprotein interactions. Nature 249: 54–57.
Clough, S.J. and Bent, A.F. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743.
Codina, A., Fernández, I., Martínez, I., Ludevid, D. and Giralt, E. 2001. Combined use of ESI-MS and UV diode-array detection for localization of disulfide bonds in proteins: application to an ?-L-fucosidase of pea. J. Peptide Res. 57: 473–482.
Codina, A., Gairi, M., Tarrago, T., Viguera, A.R., Feliz, M., Ludevid, D. and Giralt, E. 2002. 1H(N), 15N, 13CO, 13C?, 13C? assignment and secondary structure of a 20 kDa ?-L-fucosidase from pea using TROSY. J. Biomol. NMR 22: 295–296.
Dattagupta, J.K., Podder, A., Chakrabarti, C., Sen U., Mukhopadhyay, D., Dutta, S.K. and Singh, M. 1999. Refined crystal structure (2.3 Å) of a double-headed winged bean ?-chymotrypsin inhibitor and location of its second reactive site. Proteins. 35: 321–331.
de la Torre, F., Sampedro, J., Zarra, I. and Revilla, G. 2002. AtFXG1, an Arabidopsis gene encoding ?-L-Fucosidase active against fucosylated xyloglucan oligosaccharides. Plant Physiol. 128: 247–255.
Farkas, V., Hanna, R. and Maclachlan, G. 1991. Xyloglucan oligosaccharide ?-L-fucosidase activity from growing pea stems and germinating nasturtium seeds. Phytochemistry 30: 3203–3207.
Koiwa, H., Bressan, RA. and Hasegawa, PM. 1997. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 2: 379–384.
Occhiodoro, T., Beckmann, K., Morris, CP. and Hopwood, J. 1989. Human ?-L-fucosidase: complete coding sequence from cDNA clones. Biochem. Biophys. Res. Commun. 164: 439–445.
Ogata-Arakawa, M., Muramatsu, T. and Kobata, A. 1977. Alpha-L-fucosidases from almond emulsin: characterization of the two enzymes with different specificities. Arch. Biochem. Biophys. 181: 353–358.
Onesti, S., Brick, P. and Blow, D.M. 1991. Crystal structure of a Kunitz-type trypsin inhibitor from Erythrina caffra seeds. J. Mol. Biol. 217: 153–176.
Prakash, C. and Vijay, I.K. 1983. A new fluorescent tag for labeling of saccharides. Anal. Biochem. 128: 41–46.
Terada, S., Katayama, H., Noda, K., Fujimura, S. and Kimoto, E. 1994. Amino acid sequences of Kunitz family subtilisin inhibitors from seeds of Canavalia lineata. J. Biochem. 115: 397–404.
Vargas-Rechia, C., Reicher, F., Rita Sierakowski, M.R., Heyraud, A., Driguez, H. and Linart, Y. 1998. Xyloglucan octasaccharide XXLGol derived from the seeds of Hymenaea courbaril acts as a signaling molecule. Plant Physiol. 116: 1013–1021.
Wong-Madden, S.T. and Landry D. 1995. Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology 5: 19–28.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tarragó, T., Martínez, I., Torrent, M. et al. The fuc1 gene product (20 kDa FUC1) of Pisum sativum has no α-L-fucosidase activity. Plant Mol Biol 51, 877–884 (2003). https://doi.org/10.1023/A:1023053101938
Issue Date:
DOI: https://doi.org/10.1023/A:1023053101938