Abstract
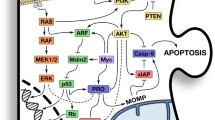
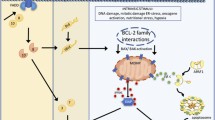
Mitochondria are potent integrators/coordinators of apoptosis signaling pathways. Indeed, under physiological conditions, the initiation of apoptosis leads to the accumulation of second messengers that converge on mitochondria. In response, these organelles undergo a membrane permeabilization, presumably due to the opening of protein channels, culminating in the release of proapoptotic proteins into the cytosol. Under pathological conditions, a failure of mitochondrial membrane permeabilization (MMP) can result in an inhibition of apoptosis and enhanced resistance to chemotherapy. Several non-mutually exclusive mechanisms may account for a defect in the execution or regulation of MMP. These include (i) alterations in gene transcription, (ii) gene mutations resulting in protein inactivation, and (iii) defects of intracellular localization. This may concern structural proteins of the permeability transition pore complex, as well as MMP regulatory proteins, such as Bax/Bcl-2 family members, p53, and cyclophilin D. Analysis of these mechanisms should improve our understanding of the basic function of mitochondria in apoptosis and help elaborate new strategies to correct MMP failure from a therapeutic perspective.
Similar content being viewed by others
REFERENCES
Kerr JF, Wyllie AH, Currie AR: Apoptosis: A basic biological phe-nomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257, 1972
Green DR, Reed JC: Mitochondria and apoptosis. Science 281:1309–1312, 1998
Kroemer G, Reed J: Mitochondrial control of cell death. Nat Med 6:513–519, 2000
Desagher S, Martinou JC: Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377, 2000
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiére J-L, Petit PX, Kroemer G: Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181:1661–1672, 1995
Marchetti P, Decaudin D, Macho A, Zamzami N, Hirsch T, Susin SA, Kroemer G: Redox regulation of apoptosis: Impact of thiol oxi-dation status on mitochondrial function. Eur J Immunol 27:289–296, 1997
Liu X, Kim CN, Yang J, Jemmerson R, Wang X: Induction of apoptic program in cell-free extracts: Requirement for dATP and cytochrome C. Cell 86:147–157, 1996
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X: Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413, 1997
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G: Mitochondrial release of caspase-2 and-9 during the apoptotic process. J Exp Med 189:381–393, 1999
Adrain C, Creagh EM, Martin SJ: Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20:6627–6636, 2001
Li LY, Luo X, Wang X: Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412:95–99, 2001
Brenner C, Kroemer G: Apoptosis. Mitochondria-The death signal integrators. Science 289:1150–1151, 2000
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K, Debatin K, Krammer P, Peter P: Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687, 1998
Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L: The mitochondrial permeability transition. Biofactors 8:273–281, 1998.
Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 341:233–249, 1999
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G: Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377, 1995
Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G: Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett. 384:53–57, 1996
Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G: Bcl-2 inhibits the mitochon-drial release of an apoptogenic protease. J Exp Med 184:1331–1342, 1996
Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G: Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281:2027–2031, 1998
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G: The permeabil-ity transition pore complex: A target for apoptosis regulation by caspases and Bcl-2-related proteins. J Exp Med 187:1261–1271, 1998
Zamzami N, Hamel CE, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G, Kroemer G: Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene 19:6342–6350, 2000
Vieira HL, Haouzi D, Hamel CE, Jacotot E, Belzacq AS, Brenner C, Kroemer G: Permeabilization of the mitochondrial inner membrane during apoptosis: Impact of the adenine nucleotide translocator. Cell Death Different 7:1146–1154, 2000
Belzacq AS, Vieira HL, Kroemer G, Brenner C: The adenine nu-cleotide translocator in apoptosis. Biochimie 84:167–176, 2002
De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F: The permeability transition pore signals apoptosis by directing Bax translocation and multimer-ization. FASEB J 16:607–609, 2002
Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D: Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem 277:44317–44326, 2002
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S: Cy-tochrome c release from mitochondria proceeds by a two-step pro-cess. Proc Natl Acad Sci USA 99:1259–1263, 2002
FaureVigny H, Heddi A, Giraud S, Chautard D, Stepien G: Expres-sion of oxidative phosphorylation genes in renal tumors and tumoral cell lines. Mol Carcinogen 16:165–172, 1996
Sebastian S, Kenkare UW: Expression of two type II-like tumor hexokinase RNAtranscripts in cancer cell lines. Tumor Biol 19:253–260, 1998
Maaser K, Grabowski P, Sutter AP, Hopfner M, Foss HD, Stein H, Berger G, Gavish M, Zeitz M, Scherubl H: Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic fac-tor in stage III colorectal cancer. Clin Cancer Res 8:3205–3209, 2002
Shinohara Y, Ishida T, Hino M, Yamazaki N, Baba Y, Terada H: Characterization of porin isoforms expressed in tumor cells. Eur J Biochem 267:6067–6073, 2000
Shiio Y, Donohoe S, Yi EC, Goodlett D, Aebersold R, Eisenman RN: Quantitative proteomic analysis of Myc oncoproteins function. EMBO J 21:5088–5096, 2002
Giraud S, Bonod-Bidaud C, Wesolowski-Louvel M, Stepien G: Ex-pression of human ANT2gene in highly proliferative cells: GRBOX, a new transcriptional element, is involved in the regulation of gly-colytic ATP import into mitochondria. J Mol Biol 281:409–418, 1998
Barath P, Luciakova K, Hodny Z, Li R, Nelson BD: The growth-dependent expression of the adenine nucleotide translocase-2 (ANT2) gene is regulated at the level of transcription and is a marker of cell proliferation. Exp Cell Res 248:583–588, 1999
Bauer MK, Schubert A, Rocks O, Grimm S: Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J Cell Biol 147:1493–1502, 1999
Fagiolari G, Sciacco M, Chiveri L, Lamperti C, Comi GP, Scarlato G, Moggio M, Prelle A: Lack of apoptosis in patients with pro-gressive external ophthalmoplegia and mutated adenine nucleotide translocator-1 gene. Muscle Nerve 26:265–269, 2002
Komaki H, Fukazawa T, Houzen H, Yoshida K, Nonaka I, Goto Y: A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmople-gia patients with mitochondrial DNA with multiple deletions. Ann Neurol 51:645–648, 2002
Elpeleg O, Mandel H, Saada A: Depletion of the other genome-mitochondrial DNA depletion syndromes in humans. J Mol Med 80:389–396, 2002
Van Goethem G, Martin JJ, Van Broeckhoven C: Progressive ex-ternal ophthalmoplegia and multiple mitochondrial DNA deletions. Acta Neurol Belg 102:39–42, 2002
Reed JC, Jurgensmeier JM, Matsuyama S: Bcl-2 family proteins and mitochondria. Biochim Biophys Acta 1366:127–137, 1998
Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, Reed JC, Andreeff M: The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol 118:521–534, 2002
Kitada S, Pedersen IM, Schimmer AD, Reed JC: Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene 21:3459–3474, 2002
Gascoyne RD, Krajewska M, Krajewski S, Connors JM, Reed JC: Prognostic significance of Bax protein expression in dif-fuse aggressive non-Hodgkin's lymphoma. Blood 90:3173–3178, 1997
Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A, Mazzone C, Panetta P, Suppo G, Masi M, Amadori S: The amount of spontaneous apop-tosis detected by bax/bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 7:7, 2002
Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC, Kroemer G: Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene 19:329–336, 2000
Soussi T, Beroud C: Assessing TP53 status in human tumours to evaluate clinical outcome. Natl Rev Cancer 1:233–240, 2001
Guimaraes DP, Hainaut P: TP53: A key gene in human cancer. Biochimie 84:83–93, 2002
Marchenko ND, Zaika A, Moll UM: Death signal-induced localiza-tion of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem 275:16202–16212, 2000
Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T: p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416:560–564, 2002
Halestrap AP, McStay GP, Clarke SJ: The permeability transition pore complex: Another view. Biochimie 84:153–166, 2002
Lin DT, Lechleiter JD: Mitochondrial targeted cyclophilin Dprotects cells from cell death by peptidyl prolyl isomerization. J Biol Chem 277:31134–31141, 2002
Costantini P, Jacotot E, Decaudin D, Kroemer G: Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst 92:1042–1053, 2000
Debatin K-M, Poncet D, Kroemer G: Chemotherapy: Targeting the mitochondrial cell death pathway. Oncogene (in press)
Belzacq AS, El Hamel C, Vieira HL, Cohen I, Haouzi D, Metivier D, Marchetti P, Brenner C, Kroemer G: Adenine nucleotide transloca-tor mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene 20:7579–7587, 2001
Terminella C, Tollefson K, Kroczynski J, Pelli J, and Cutaia M: Inhibition of apoptosis in pulmonary endothelial cells by altered pH, mitochondrial function, and ATP supply. Am J Physiol Lung Cell Mol Physiol 283:L1291–L1302, 2002
Rempel A, Mathupala SP, Griffin CA, Hawkins AL, Pedersen PL: Glucose catabolism in cancer cells: Amplification of the gene en-coding type II hexokinase. Cancer Res 56:2468–2471, 1996
Owen-Schaub LB, Zhang W, Cusack JC, et al.: Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 15:3032–3040, 1995
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, Jacks T: PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev 14:704–718, 2000
Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, El-Deiry WS: KILLER/DR5 is a DNAdamage-inducible p53-regulated death receptor gene. Nat Genet 17:141–143, 1997
Takimoto R, El-Deiry WS: Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 19:1735–1743, 2000
Contente A, Dittmer A, Koch MC, Roth J, Dobbelstein M: A poly-morphic microsatellite that mediates induction of PIG3 by p53. Nat Genet 30:315–320, 2002
Lin Y, Ma W, Benchimol S: Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat Genet 26:122–127, 2000
Okamura S, Arakawa H, Tanaka T, Nakanishi H, Ng CC, Taya Y, Monden M, Nakamura Y: p53DINP1, a p53-inducible gene, regu-lates p53-dependent apoptosis. Mol Cell 8:85–94, 2001
Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y: p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849–862, 2000
Matsuda K, Yoshida K, Taya Y, Nakamura K, Nakamura Y, Arakawa H: p53AIP1 regulates the mitochondrial apoptotic pathway. Cancer Res 62:2883–2889, 2002
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW: Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156–159, 1999
Robles AI, Bemmels NA, Foraker AB, Harris CC: APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res 61:6660–6664, 2001
Moroni MC, Hickman ES, Denchi EL, Caprara G, Colli E, Cecconi F, Muller H, Helin K: Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol 3:552–558, 2001
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N: Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058, 2000
Nakano K, Vousden KH: PUMA, a novel proapoptotic gene, is in-duced by p53. Mol Cell 7:683–694, 2001
Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM: Nega-tive regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene 20:240–251, 2001
Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM: Proline oxidase, encoded by p53-induced gene-6, catalyzes the gen-eration of proline-dependent reactive oxygen species. Cancer Res 61:1810–1815, 2001
Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B: Ferredoxin reductase af-fects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med 7:1111–1117, 2001
Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng C, Steven AC, Weinberg WC, Yuspa SH: mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and partici-pates in the apoptotic response to p53. Mol Cell Biol 22:3610–3620, 2002
Zhou M, Gu L, Li F, Zhu Y, Woods WG, Findley HW: DNA damage induces a novel p53-survivin signaling pathway regulating cell cycle and apoptosis in acute lymphoblastic leukemia cells. J Pharmacol Exp Ther 303:124–131, 2002
Miyashita T, Reed JC: Tumor suppressor p53 is a direct tran-scriptional activator of the human bax gene. Cell 80:293–299, 1995
Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS: BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849, 2002
Pohl U, Wagenknecht B, Naumann U, Weller M: p53 enhances BAK and CD95 expression in human malignant glioma cells but does not enhance CD95L-induced apoptosis. Cell Physiol Biochem 9:29–37, 1999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brenner, C., Le Bras, M. & Kroemer, G. Insights into the Mitochondrial Signaling Pathway: What Lessons for Chemotherapy?. J Clin Immunol 23, 73–80 (2003). https://doi.org/10.1023/A:1022541009662
Issue Date:
DOI: https://doi.org/10.1023/A:1022541009662