Abstract
Purpose. To investigate the influence of type and amount of excipient on the preservation of the native structure and the biologic activity of freeze-dried lysozyme and catalase.
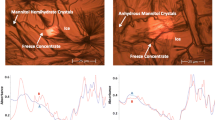
Methods. The secondary structure of protein in the dried form and in aqueous solution was obtained using second derivative infrared spectroscopy and circular dichroism spectra respectively whilst the activity was determined using bioassay.
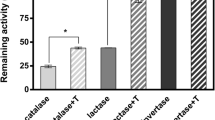
Results. Small molecular excipients (glycerol, sorbitol, 1,6-anhydroglucose, sucrose, and trehalose) were found to stabilize the activity and/or the native structure of freeze-dried lysozyme and catalase, despite the processing temperatures being above Tg′ of excipient-protein mixtures. The preservation of catalase activity required excipient to be present at a lower excipient to enzyme mass ratio than that necessary to preserve native structure in the dried form. Combining dextran with sucrose synergistically protected the native structure of catalase but preserved the activity in an additive manner.
Conclusion. The results indicate that the stabilization of catalase and lysozyme by excipients during dehydration was mainly due to water substitution rather than the formation of glass; the latter appearing not to be a prerequisite during freeze-drying.
Similar content being viewed by others
REFERENCES
T. Arakawa, S. J. Prestrelski, W. C. Kenney, and J. F. Carpenter. Factors affecting short and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 10:1–28 (1993).
M. C. Manning, K. Patel, and R. T. Borchardt. Stability of protein pharmaceuticals. Pharm. Res. 11:903–918 (1989).
S. J. Prestrelski, N. Tedeschi, T. Arakawa, and J. F. Carpenter. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 65:661–671 (1993).
J. F. Carpenter, M. J. Pikal, B. S. Chang, and T. W. Randolph. Rational design of stable lyophilized protein formulations: Some practical advice. J. Pharm. Sci. 14:969–975 (1997).
K. Tanaka, T. Takeda, and K. Miyajima. Cryoprotective effect of saccharides on denaturation of catalase by freeze-drying. Chem. Pharm. Bull. 39:1091–1094 (1991).
S. D. Allison, M. C. Manning, T. W. Randolph, K. Middleton, A. Davis, and J. F. Carpenter. Optimization of storage stability of lyophilized actin using combinations of disaccharides and dextran. J. Pharm. Sci. 89:199–214 (2000).
J. F. Carpenter, K. Izutsu, and T. W. Randolph. Freezing and drying-induced perturbations of protein structure and mechanisms of protein protection by stabilizing additives. In L. Rey and J. C. May (eds.), Freeze-drying/lyophilization of pharmaceutical and biological products, Marcel Dekker Inc., New York. 1999 pp. 123–160.
J. L. Cleland, X. Lam, B. Kendrick, J. Yang, T. H. Yang, D. Overcashier, D. Brooks, C. Hsu, and J. F. Carpenter. A specific molar ratio of stabilizer to protein is required for storage stability of a lyophilized monoclonal antibody. J. Pharm. Sci. 90:310–321 (2001).
J. F. Carpenter and J. H. Crowe. The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244–255 (1988).
W. Wang. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 203:1–60 (2000).
S. D. Allison, B. S. Chang, T. W. Randolph, and J. F. Carpenter. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem.Biophys. 365:289–298 (1999).
F. Franks, R. H. M. Hatley, and S. F. Mathias. Material science and production of shelf stable biological. Biopharm. 4:38–55 (1991).
M. J. Pikal. Mechanisms of protein stabilization during freezedrying and storage: The relative importance of thermodynamic stabilization and glassy state relaxation dynamics. In L. Rey and J. C. May (eds.), Freeze-drying/lyophilization of pharmaceutical and biological products, Marcel Dekker Inc., New York, 1999 pp.161–198.
Y. H. Liao, M. C. Brown, and G. P. Martin. Turbidimetric and HPLC assays for the determination of formulated lysozyme activity. J. Pharm. Pharmacol. 53: 549-554.
H. Aebi. Catalase. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, Academic Press, Inc., London, 1974 pp. 673–684.
K. M. Malik. 1997. Chemometric and quantum mechanical methods to analyse CD data. PhD thesis, University of London.
S. D. Allison, T. W. Randolph, M. C. Manning, K. Middleton, A. Davis, and J. F. Carpenter. Effects of drying methods and additives on structure and function of actin: Mechanism of dehydration-induced damage and its inhibition. Arch. Biochem. Biophys. 358:171–181 (1998).
A. Dong, P. Huang, and W. S. Caughey. Redox-dependent changes in ?-extended chain and turn structure cytochrome c in water solution determined by second derivative amide I infrared spectra. Biochemistry 31:182–189 (1992).
M. Gordon and J. S. Taylor. Ideal copolymers and the secondorder transitions of synthetic rubbers, I: non-crystalline copolymer. J. Appl. Chem. 2:493–498 (1952).
L. M. Her and S. L. Nail. Measurement of glass transition temperatures of free-concentrated solutes by differential scanning calorimetry. Pharm. Res. 11:54–59 (1994).
K. Griebenow and A. M. Klibanov. Lyophilization-induced reversible changes in the secondary structure of proteins. Proc. Natl. Acad. Sci. USA 92:10969–10976 (1995).
J. A. Rupley and G. Careri. Protein hydration and function. Adv. Pro. Chem. 41:38–173 (1991).
P. L. Poole. The role of hydration in lysozyme structure and activity: Relevance in protein engineering and design. J. Food Eng. 22:37–172 (1994).
K. R. Ward, G. D. Adams, H. Q. Alpar, and W. J. Irwin. Protection of the enzyme L-asparaginase during lyophilisation-a molecular modelling an approach to predict required level of lyoprotectant. Int. J. Pharm. 187:153–162 (1999).
J. F. Carpenter, S. J. Prestrelski, and A. Dong. Application of infrared spectroscopy to development of stable lyophilized protein formulations. Eur. J. Pharm. Biopharm. 45:231–238 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, YH., Brown, M.B., Quader, A. et al. Protective Mechanism of Stabilizing Excipients Against Dehydration in the Freeze-Drying of Proteins. Pharm Res 19, 1854–1861 (2002). https://doi.org/10.1023/A:1021497625645
Issue Date:
DOI: https://doi.org/10.1023/A:1021497625645