Abstract
Purpose. To develop and demonstrate a novel particle engineering technology, spray freezing into liquid (SFL), to enhance the dissolution rates of poorly water-soluble active pharmaceutical ingredients (APIs).
Methods. Model APIs, danazol or carbamazepine with or without excipients, were dissolved in a tetrahydrofuran/water cosolvent system and atomized through a nozzle beneath the surface of liquid nitrogen to produce small frozen droplets, which were subsequently lyophilized. The physicochemical properties of the SFL powders and controls were characterized by X-ray diffraction, scanning electron microscopy (SEM), particle size distribution, surface area analysis, contact angle measurement, and dissolution.
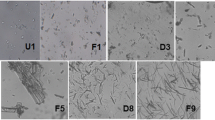
Results. The X-ray diffraction pattern indicated that SFL powders containing either danazol or carbamazepine were amorphous. SEM micrographs indicated that SFL particles were highly porous. The mean particle diameter of SFL carbamazepine/SLS powder was about 7 μm. The surface area of SFL danazol/poloxamer 407 powder was 11.04 m2/g. The dissolution of SFL danazol/poloxamer 407 powder at 10 min was about 99%. The SFL powders were free flowing and had good physical and chemical stability after being stored at 25°C/60%RH for 2 months.
Conclusions. The novel SFL technology was demonstrated to produce nanostructured amorphous highly porous particles of poorly water soluble APIs with significantly enhanced wetting and dissolution rates.
Similar content being viewed by others
REFERENCES
G. L. Amidon, H. Lnnernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
L. Bertisson. Clinical pharmacokinetics of carbamazepine. Clin. Pharmacokinet. 3:128–143 (1978).
T. Loftsson and H. Frioriksdottir. The effect of water-soluble polymers on the aqueous solubility and complexing abilities of ?-cyclodextrin. Int. J. Pharm. 163:115–121 (1998).
Y. F. Maa, P. A. Nguyen, T. Sweeney, S. J. Shire, and C. C. Hsu. Protein inhalation powders: spray drying vs. spray freeze drying. Pharm. Res. 16:249–254 (1999).
T. L. Rogers, K. P. Johnston, and R. O. Williams, III. A comprehensive review: solution-based particle formation of pharmaceutical powders by supercritical or compressed fluid CO2 and cryogenic spray-freezing technologies. Drug Dev. Ind. Pharm. 27: 1003–1015 (2001).
W. Wang. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 203:1–60 (2000).
T. H. Adams, J. P. Beck, and R. C. Menson. Novel particulate compositions. US4323478 (1982).
A. R. Briggs and T. J. Maxwell. Method of preparation of lyophilized biologic products. US3932943 (1976).
D. B. Dunn, G. J. Masavage, and H. A. Sauer. Method of freezing solution droplets and the like using immiscible refrigerants of differing densities. US3653222 (1972).
I. R. Buxton and J. M. Peach. Process and apparatus for freezing a liquid medium. US4470202 (1984).
H. A. Sauer. Method and apparatus for freeze-freeze drying. US3484946 (1969).
The Montreal Protocol on Substances That Deplete the Ozone Layer. 1987.
R. O. Williams, T. L. Rogers, and J. Liu. Study of solubility of steroids in hydrofluoroalkane propellants. Drug Dev. Ind. Pharm. 25:1227–1234 (1999).
W. R. Gombotz, M. S. Healy, L. R. Brown, and H. E. Auer. Process for producing small particles of biologically active molecules. WO90/13285 (1990).
M. I. Gusman and S. M. Johnson. Cryochemical method of preparing ultrafine particles of high-purity superconducting oxides. US4975415 (1990).
B. D. Cullity. Chemical analysis by X-ray diffraction. In B. D. Cullity (eds.), Elements of X-ray Diffraction, Addison-Wesley, Massachusetts, 2001 pp. 397–420.
S. Brunauer, P. H. Emmett, and E. Teller. Adsorption of gases in multimolecular layer. J. Am. Chem. Soc. 60:309–319 (1938).
E. Galia, E. Nicolaides, D. Horter, R. Lobenberg, C. Reppas, and J. B. Dressman. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 15:698–705 (1998).
S. Brown, G. Rowley, and J. T. Pearson. Surface treatment of the hydrophobic drug danazol to improve drug dissolution. Int. J. Pharm. 165:227–237 (1998).
B. L. Pedersen, A. Mullertz, H. Brondsted, and H. C. Kristensen. A comparison of the solubility of danazol in human and simulated gastrointestinal fluids. Pharm. Res. 17:891–894 (2000).
M. Shibata, H. Kokubo, K. Morimoto, T. Ishida, and M. Inoue. X-ray structural studies and physicochemical properties of cimetidine polymorphism. J. Pharm. Sci. 72:1436 (1983).
G. G. Liversidge and K. C. Cundy. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 125:91–97 (1995).
M. J. Lowes, M. R. Caira, A. P. Lotter, and J. G. VanderWatt. Physicochemical properties and X-ray structure studies of the trigonal polymorph of carbamazepine. J. Pharm. Sci. 76:744–752 (1987).
N. Kondo, T. Iwao, H. Masuda, K. Yamanouchi, Y. Ishihara, N. Yamada, T. Haga, Y. Ogawa, and K. Yokoyama. Improved oral absorption of a poorly water soluble drug, HO-221, by wet-bead milling producing particles in submicron region. Chem. Pharm. Bull. 41:737–740 (1993).
A. H. Lefebvre. Atomization and Sprays, Hemisphere Publishing Corp., New York, 1989.
H. R. Costantino, L. Firouzabadian, K. Hogeland, C. Wu, C. Beganski, K. G. Carrasquillo, M. Córdova, K. Griebenow, S. E. Zale, and M. A. Tracy. Protein spray-freeze drying. Effect of atomization condition on particle size and stability. Pharm. Res. 17:1374–1383 (2000).
V. Bakatselou, R. C. Oppenheim, and J. B. Dressman. Solubilization and wetting effects of bile salts on the dissolution of steroids. Pharm. Res. 8:1461–1469 (1991).
A. P. Martin. Interfacial phenomena. In A. P. Martin (ed.), Physical Pharmacy, Lea & Febiger, Philadelphia, 1993 pp. 384–386.
A. W. Adamson and A. P. Gast. Physical Chemistry of Surfaces, Wiley, New York, 1997.
V. P. Shah, Y. Tsong, P. Sathe, and J.-P. Liu. In vitro dissolution profile comparison-statistics and analysis of the similarity factor, f2. Pharm. Res. 15:889–896 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, J., Rogers, T.L., Brown, J. et al. Improvement of Dissolution Rates of Poorly Water Soluble APIs Using Novel Spray Freezing into Liquid Technology. Pharm Res 19, 1278–1284 (2002). https://doi.org/10.1023/A:1020390422785
Issue Date:
DOI: https://doi.org/10.1023/A:1020390422785