Abstract
Purpose. To delineate mechanisms associated with the corneal transport of a L-valine prodrug of an antiviral agent, acyclovir.
Method. The permeability and enzymatic hydrolysis of L-Val-ACV were evaluated using freshly excised rabbit cornea. Transport mechanism across rabbit cornea was investigated through a competitive inhibition study of L-Val-ACV with other substrates of human peptide transporter (hPepT1).
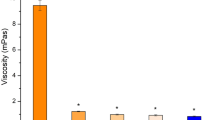
Results. L-Valyl ester of Acyclovir (L-Val-ACV) was approximately threefold more permeable across the intact rabbit cornea than acyclovir (ACV). Dipeptides, β-lactam antibiotics, and angiotensin converting enzyme (ACE) inhibitors, strongly inhibited the transport of L-Val-ACV indicating that a carrier mediated transport system specific for peptides is primarily responsible for the corneal permeation of L-Val-ACV. L-Val-ACV transport was found to be saturable (K m = 2.26 ± 0.34 mM, J max = 1.087 ± 0.05 nmoles cm− 2 min− 1), energy and pH dependent.
Conclusions. Functional evidence of an oligopeptide transport system present on the rabbit cornea has been established. The peptide transporter on the corneal epithelium may be targeted to improve the ocular bioavailability of poorly absorbed drugs.
Similar content being viewed by others
REFERENCES
D. L. Easty. Clinical Aspects of Ocular Herpes Simplex Virus Infection: Chicago Year Book Medical Publishers, 1985.
D. M. Richards, A. A. Carmine, R. N. Brogden, R. C. Heel, T. M. Speight and G. S. Avery. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 26:378–438 (1983).
J. J. Sanitato, P. A. Asbell, E. D. Varnell, G. E. Kissling, and H. E. Kaufman. Acyclovir in the treatment of herpetic stromal disease. Am. J. Ophthalmol. 98:537–547 (1984).
P. M. Hughes and A. K. Mitra. Effect of acylation on the ocular disposition of acyclovir. II: Corneal permeability and anti-HSV 1 activity of 2´-esters in rabbit epithelial keratitis. J. Ocul. Pharmacol. 9:299–309 (1993).
S. Weller, M. R. Blum, M. Doucette, T. Burnette, D. M. Cederberg, P. de Miranda, and M. L. Smiley. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single-and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595–605 (1993).
R. H. Lupia, N. Ferencz, J. J. Lertora, S. K. Aggarwal, W. J. George, and K. C. Agrawal. Comparative pharmacokinetics of two prodrugs of zidovudine in rabbits: enhanced levels of zidovudine in brain tissue. Antimicrob. Agents Chemother. 37:818–824 (1993).
L. M. Beauchamp, G. F. Orr, P. D. Miranda, and T. A. Krenitsky. Amino acid ester prodrugs of Acyclovir. Antiviral. Chem. Chemotherap. 3:157–164 (1992).
H. K. Han, D. M. Oh, and G. L. Amidon. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm. Res. 15:1382–1386 (1998).
P. V. Balimane, I. Tamai, A. Guo, T. Nakanishi, H. Kiteda, F. H. Leibach, A. Tsuji, and P. J. Sinko. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem. Biophys. Res. Commun. 250:246–251 (1998).
R. L. de Vrueh, P. L. Smith, and C. P. Lee. Transport of L-valine-acyclovir via the oligopeptide transporter in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 286:1166–1170 (1998).
X. Zhou, M. Thamotharan, A. Gangopadhyay, C. Serdikoff, and A. A. Adibi. Characterization of an oligopeptide transporter in renal lysosomes. Biochim. Biophys. Acta. 1466:372–378 (2000).
H. Saito, M. Okuda, T. Terada, S. Sasaki, and K. Inui. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J. Pharmacol. Exp. Ther. 275:1631–1637 (1995).
V. H. Lee. Membrane transporters. Eur. J. Pharm. Sci. 11Suppl 2:S41–50 (2000).
A. Tsuji and I. Tamai. Carrier-mediated intestinal transport of drugs. Pharm. Res. 13:963–977 (1996).
D. M. Oh, H. K. Han, and G. L. Amidon. Drug transport and targeting. Intestinal transport. Pharm. Biotechnol. 12:59–88 (1999).
I. Tamai and A. Tsuji. Transporter-mediated permeation of drugs across the blood-brain barrier. J. Pharm. Sci. 89:1371–1388 (2000).
B. S. Anand and A. K. Mitra. Ocular Disposition of Acyclovir Analogs: Aqueous Stability, Ocular Tissue Hydrolysis and Transport Characteristics. AAPSPharmSci (supplement) 3 (2001).
S. K. Wu, D. K. Ann, and V. H. Lee. Molecular Evidence for the Existence of Dipeptide Transporters in Rabbit Cornea and Conjunctiva. AAPSPharmSci (supplement). 3 (2001).
A. Guo, P. Hu, P. V. Balimane, F. H. Leibach, and P. J. Sinko. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 289:448–454 (1999).
C. S. Dias, B. S. Anand, and A. K. Mitra. Effect of Mono and Di Acylation on the ocular disposition of Ganciclovir: Physicochemical Properties, Ocular Bioreversion and Antiviral Activity of short chain Ester Prodrugs. J. Pharm. Sci. 91:660–668 (2002).
R. V. Tak, D. Pal, H. Gao, S. Dey, and A. K. Mitra. Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J. Pharm. Sci. 90:1505–1515 (2001).
C. Pham-Huy, F. Stathoulopoulou, P. Sandouk, J. M. Scherrmann, S. Palombo, and C. Girre. Rapid determination of valaciclovir and acyclovir in human biological fluids by high-performance liquid chromatography using isocratic elution. J. Chromatogr. B Biomed. Sci. Appl. 732:47–53 (1999).
I. Tamai, T. Nakanishi, K. Hayashi, T. Tesao, Y. Sai, T. Shirago, K. Miyamoto, E. Takaeda, H. Higashida, and A. Tsuji. The predominant contribution of oligopeptide transporter PepT1 to intestinal absorption of beta-lactam antibiotics in the rat small intestine. J. Pharm. Pharmacol. 49:796–801 (1997).
T. Terada, H. Saito, M. Mukai, and K. Inui. Recognition of betalactam antibiotics by rat peptide transporters, PEPT1 and PEPT2, in LLC-PK1 cells. Am. J. Physiol. 273:F706–F711 (1997).
M. Sugawara, W. Huang, Y. J. Fei, F. H. Leibach, V. Ganapathy, and M. E. Ganapathy. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 89:781–789 (2000).
M. E. Ganapathy, M. Brandsch, P. D. Prasad, V. Ganapathy, and F. H. Leibach. Differential recognition of beta-lactam antibiotics by intestinal and renal peptide transporters, PEPT1 and PEPT2. J. Biol. Chem. 270:25672–25677 (1995).
THE MERCK INDEX Twelfth Edition. In: S. Budavari, (ed.), Merck Research Laboratories, Division of MERCK & CO., Inc., 1996.
P. M. Hughes, R. Krishnamoorthy, and A. K. Mitra. Effect of acylation on the ocular disposition of acyclovir. I: Synthesis, physicochemical properties, and antiviral activity of 2'-esters. J. Ocul. Pharmacol. 9:287–297 (1993).
K. C. Meadows and J. B. Dressman. Mechanism of acyclovir uptake in rat jejunum. Pharm. Res. 7:299–303 (1990).
G. I. Henderson, Z. Q. Hu, R. F. Johnson, A. B. Perez, Y. Yang, and S. Schenker. Acyclovir transport by the human placenta. J. Lab. Clin. Med. 120:885–892 (1992).
K. Inui, T. Terada, S. Masuda, and H. Saito. Physiological and pharmacological implications of peptide transporters, PEPT1 and PEPT2. Nephrol Dial Transplant. 15:11–13 (2000).
R. Liang, Y. J. Fei, P. D. Prasad, S. Ramamoorthy, H. Han, T. L. Yang-Feng, M. A. Hedizer, V. Ganapathy, and F. H. Leibach. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 270: 6456–6463 (1995).
N. Lister, P. D. Bailey, I. D. Collier, C. A. Boyd, and J. R. Bronk. The influence of luminal pH on transport of neutral and charged dipeptides by rat small intestine, in vitro. Biochim. Biophys. Acta. 1324:245–250 (1997).
P. Balimane and P. Sinko. Effect of ionization on the variable uptake of valacyclovir via the human intestinal peptide transporter (hPepT1) in CHO cells. Biopharm. Drug. Dispos. 21:165–174 (2000).
M. Lucas. Determination of acid surface pH in vivo in rat proximal jejunum. Gut 24:734–739 (1983).
S. Nussberger and M. A. Hediger. How peptides cross biological membranes. Exp. Nephrol. 3:211–218 (1995).
Rights and permissions
About this article
Cite this article
Anand, B.S., Mitra, A.K. Mechanism of Corneal Permeation of L-Valyl Ester of Acyclovir: Targeting the Oligopeptide Transporter on the Rabbit Cornea. Pharm Res 19, 1194–1202 (2002). https://doi.org/10.1023/A:1019806411610
Issue Date:
DOI: https://doi.org/10.1023/A:1019806411610