Abstract
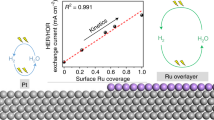
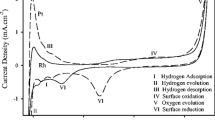
Using Auger electron spectroscopy and electrochemistry we confirm that ruthenium is spontaneously deposited on platinum single-crystal faces from ruthenium(III) chloride solutions in perchloric acid. The deposit is catalytically active towards methanol present in electrolytic media. On the Pt(111) surface - the most active catalyst for the oxidation process - the data show that the packing density of ruthenium is 9.9±1.5% and increases with ruthenium concentration in the electrolytic bath, confirming the previous electrochemical coverage estimate. In the most favorable instances the ruthenium enhancement factor, the current ratio due to methanol oxidation with and without ruthenium on the surface, is equal to15 (at 0.490 V vs. RHE reference on Pt(111)). We believe this study is an important addition to our long-range efforts in investigating surface structure effects in platinum-ruthenium heterogeneous electrocatalysis.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Chrzanowski, W., Kim, H. & Wieckowski, A. Enhancement in methanol oxidation by spontaneously deposited ruthenium on low-index platinum electrodes. Catalysis Letters 50, 69–75 (1998). https://doi.org/10.1023/A:1019042329841
Issue Date:
DOI: https://doi.org/10.1023/A:1019042329841