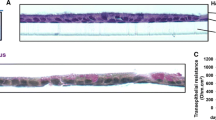
Abstract
Glycyrrhetinic acid derivatives are reported to be nasal absorption promoters (1). Effects of ammonium glycyrrhizinate (AMGZ) on the in vitro morphology of ovine nasal mucosa were therefore examined by light and electron microscopy. Nasal mucosa was stripped from the submucosa and mounted in Ussing chambers. Exposure of the apical surface to 2% ammonium glycyrrhizinate (24 mM) for 90 min caused no histopathological changes to the nasal epithelium. Epithelial integrity remained intact as evidenced by the continued presence of morphologically intact junctional complexes. No sloughing of the epithelial layer from the basement membrane was observed, and cilia and microvilli were not affected by treatment with AMGZ. The results indicate that short-term exposure in vitro to ammonium glycyrrhizinate caused no overt morphological damage to ovine nasal mucosa.
Similar content being viewed by others
REFERENCES
M. Mishima, S. Okada, Y. Wakita, and M. Nakano. Promotion of nasal absorption of insulin by glycyrrhetinic acid derivatives. I. J. Pharmacobio. Dyn. 12:31–36 (1989).
A. E. Pontiroli and G. Pozza. Intranasal drug delivery: Potential advantages and limitations from a clinical pharmacokinetic perspective. Clin. Pharmacokin. 17:299–307 (1989).
A. E. Pontiroli and G. Pozza. Intranasal administration of peptide hormones: Factors affecting transmucosal absorption. Diabetic Med. 7:770–774 (1990).
V. H. L. Lee. Protease inhibitors and penetration enhancers as approaches to modify peptide absorption. J. Control. Release 13:213–223 (1990).
L. Illum. Nasal delivery of peptides and proteins. Tibtech. 9:284–289 (1991).
C. McMartin, L. E. F. Hutchinson, R. Hyde, and G. E. Peters. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 76:535–540 (1987).
K. S. E. Su, D. C. Howey, K. M. Campanale, and J. Q. Oeswein. Intranasal administration of human sodium insulin (HSI) in rats, dogs and humans: Absorption and possible mechanisms. Diabetes 35:A64 (1986).
D. T. O'Hagan, H. Critchley, N. F. Farraj, A. Fisher, B. R. Johansen, S. S. Davis, and L. Illum. Nasal absorption enhancers for biosynthetic human growth hormone in rats. Pharm. Res. 7:772–776 (1990).
R. D. Ennis, L. Borden, and W. A. Lee. The effects of permeation enhancers on the surface morphology of rat nasal mucosa: A scanning electron microscopy study. Pharm. Res. 7:468–475 (1990).
P. Tengamnuay and A. K. Mitra. Bile salt-fatty acid mixed micelles as nasal absorption promoters of peptides. II. In vivo nasal absorption of insulin in rats and effects of mixed micelles on the morphological integrity of the nasal mucosa. Pharm. Res. 7:370–375 (1990).
V. H. L. Lee, A. Yamamoto, and U. Bhaskar Kompella. Mucosal penetration enhancers of peptide and protein drug absorption. Crit. Rev. Ther. Drug Carrier Syst. 8:91–192 (1991).
S. J. Hersey and R. T. Jackson. Effect of bile salts on nasal permeability in vitro. J. Pharm. Sci. 76:876–879 (1987).
M. A. Wheatley, J. Dent, E. B. Wheeldon, and P. L. Smith. Nasal drug delivery: An in vitro characterization of transepithelial electrical properties and fluxes in the presence or absence of enhancers. J. Control. Release 8:167–177 (1988).
P. M. Reardon, C. H. Gochoco, K. L. Audus, G. Wilson, and P. L. Smith. In vitro transport across ovine mucosa: Effects of ammonium glycyrrhizinate on electrical properties and permeability of growth hormone releasing peptide, mannitol, and lucifer yellow. Pharm. Res. 10:553–561 (1993).
P. L. Smith and M. Field. In vitro secretory effect of trifluoperazine and other neuroleptics in rabbit and human small intestine. Gastroenterology 78:1545–1553 (1980).
Y. W. Chien, K. S. E. Su, and S.-F. Chang. Nasal systemic drug delivery. In Drugs Pharm. Sci. Vol. 39, 1989.
N. Mygind. Nasal Allergy, Blackwell Scientific, London, 1979, pp. 3–38.
A. N. Fisher. Absorption across the nasal mucosa of animal species: Compounds applied and mechanisms involved. In G. G. Gibson (eds.), Progress in Drug Metabolism, Taylor and Francis, New York, 1990, pp. 87–145.
J. A. Popp, N. A. Monteiro-Riviere, and J. T. Martin. Ultrastructure of the rat nasal mucosa. In C. S. Barrow (eds.), Toxicology of the Nasal Passages, Hemisphere, Washington, DC, 1986, pp. 37–49.
P. C. Braga, G. Piatti, A. Bernini, and M. Dal Sasso. Topical tolerability of calcitonin nasal preparation, its excipients and sodium taurocholate studied by SEM and mucociliary transport velocity. Drug Invest. 4 (1):40–46 (1992).
E. L. LeCluyse, L. E. Appel, and S. C. Sutton. Relationship between drug absorption enhancing activity and membrane perturbing effects of acylcarnitines. Pharm. Res. 8:84–87 (1991).
S. G. Chandler, L. Illum, and N. W. Thomas. Nasal absorption in the rat. A method to demonstrate the histological effects of nasal formulations. Int. J. Pharm. 70:19–27 (1991).
W. A. J. J. Hermens, M. J. M. Deurloo, S. G. Romeyn, J. C. Verhoef, and F. W. H. M. Merkus. Nasal absorption enhancement of 17-β-estradiol by dimethyl-β-cyclodextrin in rabbits and rats. Pharm. Res. 7:500–503 (1990).
R. De Ponti and E. Lardini. Use of chemical enhancers for nasal drug delivery. Drug Dev. Indust. Pharm. 17:1419–1436 (1991).
G. S. Gordon, A. C. Moses, R. D. Silver, J. S. Flier, and M. C. Carey. Nasal absorption of insulin: Enhancement by hydrophobic bile salts. Proc. Natl. Acad. Sci. USA 82:7419–7423 (1985).
A. C. Moses, G. S. Gordon, M. C. Carey, and J. S. Flier. Insulin administered intranasally as an insulin-bile salt aerosol. Diabetes 32:1040–1047 (1983).
I. Andersen and D. F. Proctor. Measurement of nasal mucociliary clearance. Eur. J. Resp. Dis. 64:37–40 (1983).
J. H. Sellin and R. DeSoignie. Rabbit proximal colon: A distinct transport epithelium. Am. J. Physiol. 246:G603–G610 (1984).
B. Matuszewska, G. G. Liversidge, F. Ryan, J. Dent, and P. L. Smith. In vitro study of intestinal absorption and metabolism of 8-L-arginine vasopressin and its analogues. Int. J. Pharm. 46:1110–1120 (1988).
D. Cremaschi, C. Rossetti, M. T. Draghetti, C. Manzoni, and V. Aliverti. Active transport of polypeptides in rabbit respiratory nasal mucosa. J. Control. Release 13:319–320 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reardon, P.M., Wall, D.A., Hart, T.K. et al. Lack of Effect of Ammonium Glycyrrhizinate on the Morphology of Ovine Nasal Mucosa in Vitro . Pharm Res 10, 1301–1307 (1993). https://doi.org/10.1023/A:1018965612638
Issue Date:
DOI: https://doi.org/10.1023/A:1018965612638