Abstract
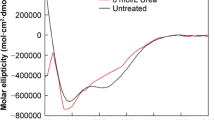
The stability of insulin and its acyl derivatives in the small intestine was examined in vitro. When these compounds were incubated in small intestinal fluid at 37°C, proteolysis of monoacyl insulins was reduced by increasing the carbon number of the fatty acid attached to Phe-B1 of the insulin molecule. In contrast, Phe-B1 and Lys-B29 diacylated insulins were more susceptible to hydrolysis than native insulin. Similar results were obtained using homogenates of the small intestinal mucosa, although the extent of the contribution of acylation to insulin degradation differed. The mechanism of the accelerated insulin proteolysis by diacylation was studied by circular dichroism (CD). The negative maxima at 270 nm in the CD spectra were attenuated for the diacyl derivatives, indicating that insulin association was inhibited by diacylation. Therefore, the increased proportion of monomers available for insulin proteolysis represents a main factor that makes diacyl derivatives unstable.
Similar content being viewed by others
REFERENCES
A. Ginsburg and H. K. Schachman. Studies on the enzymatic breakdown of proteins. I. Action of chymotrypsin on insulin. J. Biol. Chem. 235:108–114 (1960).
R. J. Schilling and A. K. Mitra. Degradation of insulin by trypsin and alpha-chymotrypsin. Pharm. Res. 8:721–727 (1991).
V. H. L. Lee, and A. Yamamoto. Penetration and enzymatic barriers to peptide and protein absorption. Adv. Drug Delivery Rev. 4:171–207 (1990).
M. Kidron, H. Bar-On, E. M. Berry and E. Ziv. The absorption of insulin from various regions of the rat intestine. Life Sci. 31:2837–2841 (1982).
S. Fujii, T. Yokoyama, K. Ikegaya, F. Sato and N. Yokoo. Promoting effect of the new chymotrypsin inhibitor FK-448 on the intestinal absorption of insulin in rats and dogs. J. Pharm. Pharmacol. 37:545–549 (1985).
C. Weingarten, A. Moufti, J. Delattre, F. Puisieux and P. Couvreur. Protection of insulin from enzymatic degradation by its association to liposomes. Int. J. Pharm. 26:251–257 (1985).
M. Shichiri, Y. Shimizu, Y. Yoshida, R. Kawamori, M. Fukuchi, Y. Shigeta and H. Abe. Enteral absorption of water-in-oil-in water insulin emulsions in rabbits. Diabetologia 10:317–321 (1974).
S. Muranishi, A. Sakai, K. Yamada, M. Murakami, K. Takada and Y. Kiso. Lipophilic peptides: Synthesis of lauroyl thyrotropin-releasing hormone and its biological activity. Pharm. Res. 8:649–652 (1991).
T. Tenma, E. Yodoya, S. Tashima, T. Fujita, M. Murakami, A. Yamamoto and S. Muranishi. Development of new lipophilic derivatives of tetragastrin: Physicochemical characteristics and intestinal absorption of acyltetragastrin derivatives in rats. Pharm. Res. 10:1488–1492 (1993).
M. Hashimoto, K. Takada, Y. Kiso and S. Muranishi. Synthesis of palmitoyl derivatives of insulin and their biological activities. Pharm. Res. 6:171–176 (1989).
K. Yamada, M. Murakami, A. Yamamoto, K. Takada and S. Muranishi. Improvement of intestinal absorption of thyrotropin-releasing hormone by chemical modification with lauric acid. J. Pharm. Pharmacol. 44:717–721 (1992).
M. Hashizume, T. Douen, M. Murakami, A. Yamamoto, K. Takada and S. Muranishi. Improvement of large intestinal absorption of insulin by chemical modification with palmitic acid in rats. J. Pharm. Pharmacol. 44:555–559 (1992).
D. G. Lindsay and S. Shall. Acetoacetylation of insulin. Biochem. J. 115:587–595 (1969).
H. Zahn, D. Brandenburg and H.-G. Gattner. Molecular basis of insulin action: Contributions of chemical modifications and synthetic approaches. Diabetes 21:468–475 (1972).
K. Takada and Y. Ushirogawa. Effect of pH, dietary proteins and trypsin inhibitors on the hydrolytic rate of human granulocyte colony-stimulating factor (G-CSF) by rat digestive enzymes. J. Pharmacobio-Dyn. 14:363–370 (1991).
A. Yamamoto, E. Hayakawa and V. H. L. Lee. Insulin and proinsulin proteolysis in mucosal homogenates of the albino rabbit: Implications in peptide delivery from nonoral routes. Life Sci. 47:2465–2474 (1990).
M. Morishita, I. Morishita, K. Takayama, Y. Machida and T. Nagai. Novel oral microspheres of insulin with protease inhibitor protecting from enzymatic degradation. Int. J. Pharm. 78:1–7 (1992).
I. Morishita, M. Morishita, K. Takayama, Y. Machida and T. Nagai. Hypoglycemic effect of novel oral microspheres of insulin with protease inhibitor in normal and diabetic rats. Int. J. Pharm. 78:9–16 (1992).
M. J. Ettinger and S. N. Timasheff. Optical activity of insulin. I. On the nature of the circular dichroism bands. Biochemistry 10:824–831 (1971).
J. Goldman and F. H. Carpenter. Zinc binding, circular dichroism, and equilibrium sedimentation studies on insulin (bovine) and several of its derivatives. Biochemistry 13:4566–4574 (1974).
T. L. Blundell, J. F. Cutfield, S. M. Cutfield, E. J. Dodson, G. G. Dodson, D. C. Hodgkin and D. A. Mercola. Three-dimensional atomic structure of insulin and its relationship to activity. Diabetes 21:492–505 (1972).
F-y. Liu, D. O. Kildsig and A. K. Mitra. Insulin aggregation in aqueous media and its effect on alpha-chymotrypsin-mediated proteolytic degradation. Pharm. Res. 8:925–929 (1991).
Y. Li, Z. Shao and A. K. Mitra. Dissociation of insulin oligomers by bile salt micelles and its effect on α-chymotrypsin-mediated proteolytic degradation. Pharm. Res. 9:864–869 (1992).
Z. Shao, Y. Li, R. Krishnamoorthy, T. Chermak and A. K. Mitra. Differential effects of anionic, cationic, nonionic, and physiologic surfactants on the dissociation, α-chymotryptic degradation, and enteral absorption of insulin hexamers. Pharm. Res. 10:243–251 (1993).
J. Brange, U. Ribel, J. F. Hansen, G. Dodson, M. T. Hansen, S. Havelund, S. G. Melberg, F. Norris, K. Norris, L. Snel, A. R. Sørensen and H. O. Voigt. Monomeric insulins obtained by protein engineering and their medical implications. Nature 333:679–682 (1988).
E. L. Smith, R. L. Hill and A. Borman. Activity of insulin degraded by leucine aminopeptidase. Biochim. Biophys. Acta 29:207–208 (1958).
R. A. Roth. Bacitracin: An inhibitor of the insulin degrading activity of glutathione-insulin transhydrogenase. Biochem. Biophys. Res. Commun. 98:431–438 (1981).
R. E. Stratford, Jr. and V. H. L. Lee. Aminopeptidase activity in homogenates of various absorptive mucosae in the albino rabbit: Implications in peptide delivery. Int. J. Pharm. 30:73–82 (1986).
P. Kopečková, K. Ikesue, L. Fornŭsek and J. Kopeček. Cleavage of peptide bonds by guinea pig brush border membrane enzymes. Proc. Int. Symp. Control. Rel. Bioact. Mat. 17:130–131 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Asada, H., Douen, T., Mizokoshi, Y. et al. Stability of Acyl Derivatives of Insulin in the Small Intestine: Relative Importance of Insulin Association Characteristics in Aqueous Solution. Pharm Res 11, 1115–1120 (1994). https://doi.org/10.1023/A:1018928613837
Issue Date:
DOI: https://doi.org/10.1023/A:1018928613837