Abstract
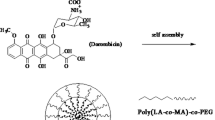
Purpose. Doxorubicin was chemically conjugated to a terminal end group of poly(D,L-lactic-co-glycolic acid) [PLGA] and the doxorubicin-PLGA conjugate was formulated into nanoparticles to sustain the release of doxorubicin.
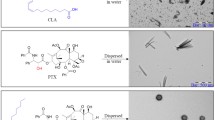
Methods. A hydroxyl terminal group of PLGA was activated by p-nitrophenyl chloroformate and reacted with a primary amine group of doxorubicin for conjugation. The conjugates were fabricated into ca. 300 nm size nanoparticles by a spontaneous emulsion-solvent diffusion method. The amount of released doxorubicin and its PLGA oligomer conjugates was quantitated as a function of time. The cytotoxicity of the released species was determined using a HepG2 cell line.
Results. Loading efficiency and loading percentage of doxorubicin-PLGA conjugate within the nanoparticles were 96.6% and 3.45 (w/w) %, respectively while those for unconjugated doxorubicin were 6.7% and 0.26 (w/w) %, respectively. Both formulation parameters increased dramatically due to the hydrophobically modified doxorubicin by the conjugation of PLGA. The nanoparticles consisting of the conjugate exhibited sustained release over 25 days, whereas those containing unconjugated free doxorubicin showed rapid doxorubicin release in 5 days. A mixture of doxorubicin and its PLGA oligomer conjugates released from the nanoparticles had comparable IC50 value in a HepG2 cell line compared to that of free doxorubicin. Sustained drug release was attributed to the chemical degradation of conjugated PLGA backbone, which permitted water solubilization and subsequent release of doxorubicin conjugated PLGA oligomers into the medium.
Conclusions. The conjugation approach of doxorubicin to PLGA was potentially useful for nanoparticle formulations that require high drug loading and sustained release. The doxorubicin-PLGA oligomer conjugate released in the medium demonstrated a slightly lower cytotoxic activity than free doxorubicin in a HepG2 cell line.
Similar content being viewed by others
REFERENCES
R. Duncan. Drug-polymer conjugates: potential for improved chemotherapy. Anti-cancer Drugs 3:175–210 (1992).
H. Maeda, M. Ueda, T. Morinaga, and T. Matsumoto. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J. Med. Chem. 28:455–461 (1985).
T. Minko, P. Kopeckova, V. Pozharov, and J. Kopecek. HPMA copolmer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J. Contr. Rel. 54:223–233 (1998).
H. Maeda and Y. Matsumura. Tumoritropic and lymphotropic principles of macromolecular drugs. CRC Crit. Rev. Ther. Drug Carrier Sys. 6:193–210 (1989).
L. W. Seymour. Passive tumor targeting of soluble macromolecules and drug conjugates. CRC Crit. Rev. Ther. Drug Carrier Sys. 9:132–187 (1992).
V. Omelyanenko, P. Kopeckova, C. Gentry, and J. Kopecek. Targetable HPMA copolymer-adriamycin conjugates. Recognition, internalization, and subcellular fate. J. Contr. Rel. 53:25–37 (1998).
M. Yokoyama, G. S. Kwon, T. Okano, Y. Sakurai, T. Seto, and K. Kataoka. Preparation of micelle-forming polymer-drug conjugates. Bioconjugate Chem. 3:295–301 (1992).
J. Leroux, E. Doelker, and R. Gurny. Microencapsulation: Methods and Industrial Applications, S. Benita Ed., Marcel Dekker, New York, 535–576 (1996).
P. Couvreur and C. Vauthier. Polyalkylcyanoacrylate nanoparticles as drug carrier: present state and perspectives. J. Contr. Rel. 17:187–198 (1991).
Y. Morimoto, K. Sugibayashi, and Y. Kato. A antitumor effect of microsphere-entrapped adriamycin of liver metastasis of AH 7974 cells in rats. Chem. Pharm. Bull. 29:1433–1439 (1981).
A. A. Gabizon, Y. Barenholz, and M. Bialer. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharm. Res. 10:703–708 (1993).
K. Yachi, H. Kikuchi, N. Suzuki, R. Atsumi, M. Aonuma, and Y. Kawato. Pharmaceutical and biological properties of doxorubicin encapsulated in liposomes (L-ADM): the effect of repeated administration on the systemic phagocytic activity and pharmacokinetics. Biopharm. Drug Dispos. 16:653–667 (1995).
J. E. Oh, Y. S. Nam, K. H. Lee, and T. G. Park. Conjugation of drug to poly(D,L-lactic co-glycolic acid) for controlled release from biodegradable microspheres. J. Contr. Rel. 57:269–280 (1999).
H. Fessi, F. Puisieux, J. P. Devissaguet, N. Ammoury, and S. Benita. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 55:R1–R4 (1989).
R. I. Freshney. Measurement of Viability and Cytotoxicity, Chapter 19 in Culture of Animal Cells, Third Edition., Wiley-Liss Inc., New York, 1994, pp. 287–307.
J. Kreuter. Nanoparticle-based drug delivery systems. J. Contr. Rel. 16:169–171 (1991).
T. G. Park. Degradation of poly(D,L-lactic)microsphere: effect of molecular weight. J. Contr. Rel. 30:161–173 (1994).
A. M. Reed and D. K. Gilding. Biodegradable polymers for use in surgery-poly(glycolci)/poly(lactic acid) homo and copolymers. 2. In vitro degradation, Polymer 22:494–498 (1981).
E. B. Yang, W. Y. Tang, K. Zhang, L. Y. Cheng, and P. O. Mack. Norcantharidin inhibits growth of human HepG2 cell-transplanted tumor in nude mice and prolongs host survival. Cancer Letter 117:93–98 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, H.S., Oh, J.E., Lee, K.H. et al. Biodegradable Nanoparticles Containing Doxorubicin-PLGA Conjugate for Sustained Release. Pharm Res 16, 1114–1118 (1999). https://doi.org/10.1023/A:1018908421434
Issue Date:
DOI: https://doi.org/10.1023/A:1018908421434