Abstract
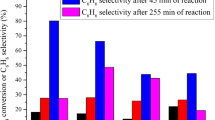
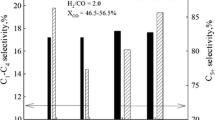
The effects of CO2, CO and H2 co-reactants on CH4 pyrolysis reactions catalyzed by Mo/H-ZSM-5 were investigated as a function of reaction temperatures and co-reactant and CH4 concentrations. Total CH4 conversion rates were not affected by CO2 co-reactants, except at high CO2 pressures, which led to the oxidation of the active MoC x species, but CH x intermediates formed in rate-determining C–H bond activation steps increasingly formed CO instead of hydrocarbons as CO2 concentrations increased. CO formation rates increased with increasing CO2 partial pressure; all entering CO2 molecules reacted with CH4 within the catalyst bed to form two CO molecules at 950-1033 K. In contrast, hydrocarbon formation rates decreased linearly with increasing CO2 partial pressure and reached undetectable levels at CO2/CH4 ratios above 0.075 at 950 K. CO formation continued for a short period of time at these CO2/CH4 molar ratios, but then all catalytic activity ceased, apparently as a result of the conversion of active carbide structures to MoO x . The removal of CO2 from the CH4 stream led to gradual catalyst reactivation via reduction-carburization processes similar to those observed during the initial activation of MoO x /H-ZSM-5 precursors in CH4. The CO2/CH4 molar ratios required to inhibit hydrocarbon synthesis were independent of CH4 pressure because of the first-order kinetic dependencies of both CH4 and CO2 activation steps. These ratios increased from 0.075 to 0.143 as reaction temperatures increased from 950 to 1033 K. This temperature dependence reflects higher activation energies for reductant (CH4) than for oxidant (CO2) activation, leading to catalyst oxidation at higher relative oxidant concentrations as temperature increases. The scavenging of CH x intermediates by CO2-derived species leads also to lower chain growth probabilities and to a significant inhibition of catalyst deactivation via oligomerization pathways responsible for the formation of highly unsaturated unreactive deposits. CO co-reactants did not influence the rate or selectivity of CH4 pyrolysis reactions on Mo/H-ZSM-5; therefore, CO formed during reactions of CO2/CH4 mixtures are not responsible for the observed effects of CO2 on reaction rates and selectivities, or in catalyst deactivation rates during CH4 reactions. H2 addition studies showed that H2 formed during CH4/CO2 reactions near the bed inlet led to inhibited catalyst deactivation in downstream catalyst regions, even after CO2 co-reactants were depleted.
Similar content being viewed by others
References
J. Lunsford, Catal. Today 6 (1996) 235.
L. Guczi, R.A. VanSanten and K.V. Sarma Catal. Rev. 38 (1996) 249.
J.B. Claridge, M.L.H. Green, S.C. Tsang and A.P.E. York, Appl. Catal. 89 (1992) 103.
M.L.H. Green, S.C. Tsang, P.D.F. Vernon and A.P.E. York, Ind. Eng. Chem. Res. 32 (1993) 1030.
L.S. Wang, L.X. Tao, M.S. Xie, G.F. Xu, J.S. Huang and Y.D. Xu, Catal. Lett. 21 (1993) 35.
B.M. Weckhuysen, D.J. Wang, M.P. Roseynek and J.H. Lunsford, J. Catal. 175 (1998) 338.
C.L. Zhang, S. Li, Y. Yuan, W. Zhang, T. Wu and L.W. Lin, Catal. Lett. 56 (1998) 207.
S.T. Liu, L.S. Wang, R. Ohnishi and M. Ichikawa, J. Catal. 181 (1999) 175.
W. Ding, G.D. Meitzner, D.O. Marler and E. Iglesia, J. Phys. Chem. B 105 (2001) 3928.
W. Ding, S. Li, G.D. Meitzner and E. Iglesia, J. Phys. Chem. B 105 (2001) 506.
R.W. Borry III, Y.H. Kim, A. Huffsmith, J.A. Reimer and E. Iglesia, J. Phys. Chem. B 103 (1999) 5787.
F. Solymosi, A. Erdohelyi and A. Szoke, Catal. Lett. 32 (1995) 43.
A. Szoke and F. Solymosi, Appl. Catal. A: General 142 (1996) 361.
F. Solymosi, J. Cserenyi, A. Szoke, T. Bansagi and A. Oszko, J. Catal. 165 (1997) 156.
S.-T. Wong, Y. Xu, S. Liu, L. Wang and X. Guo, Catal. Lett. 38 (1996) 39.
D. Wang, J.H. Lunsford and M.P. Rosynek, Topics Catal. 3 (1996) 289.
J.Z. Zhang, M.A. Long and R.F. Howe, Catal. Today 44 (1999) 293.
F. Solymosi, A. Szoke and J. Cserenyi, Catal. Lett. 39 (1996) 157.
Y.D. Liu, J. Lin and K.L. Tan, Catal. Lett. 50 (1998) 165.
R. Ohnishi, S. Liu, Q. Dong, L. Wang and M. Ichikawa, J. Catal. 182 (1999) 92.
D. Wang, J.H. Lunsford and M.P. Rosynek, J. Catal. 169 (1997) 347.
L. Wang, L. Tao, M. Xie, G. Xu, J. Huang and Y. Xu, Catal. Lett. 21 (1993) 35.
S. Liu, L. Wang, Q. Dong, R. Ohnishi and M. Ichikawa, Chem. Commun. (1998) 1217.
L. Wang, R. Ohnishi and M. Ichikawa, Catal. Lett. 62 (1999) 29.
L. Wang, R. Ohnishi and M. Ichikawa, J. Catal. 190 (2000) 276.
R. Ohnishi, L. Xu, K. Issoh and M. Ichikawa, Stud. Surf. Sci. Catal. (2001) 136.
J.R. Rostrup-Nielsen in: Catalysis: Science and Technology, eds. J.R. Anderson and M. Boudart (Springer, Berlin, 1984) p. 1.
D.R. Stull, F. Edgar, J. Westrum and G.C. Sinke, in: The Chemical Thermodynamics of Organic Compounds (Robert E. Krieger Publishing, Malabar, FL, 1987).
J.B. Claridge, A.P.E. York, A.J. Brungs, C. Marquez-Alvarez, J. Sloan, S.C. Tsang and M.L.H. Green, J. Catal. 180 (1998) 85.
C. Gueret, M. Daroux and F. Billaud, Chem. Eng. Sci. 52 (1997) 815.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, Z., Nutt, M.A. & Iglesia, E. The Effects of CO2, CO and H2 Co-Reactants on Methane Reactions Catalyzed by Mo/H-ZSM-5. Catalysis Letters 81, 271–279 (2002). https://doi.org/10.1023/A:1016553828814
Issue Date:
DOI: https://doi.org/10.1023/A:1016553828814