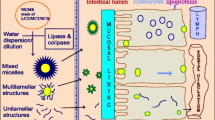
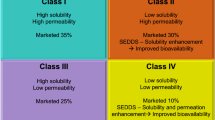
Abstract
Purpose. This review highlights the state-of-the-art in pharmaceutical microemulsions with emphasis on self-emulsifying systems, from both a physical and biopharmaceutical perspective. Although these systems have several pharmaceutical applications, this review is primarily focused on their potential for oral drug delivery and intestinal absorption improvement.
Methods. Physicochemical characteristics and formulation design based on drug solubility and membrane permeability are discussed.
Results. Case studies in which lipid microemulsions have successfully been used to improve drug solubilization/dissolution and/or intestinal absorption of poorly absorbed drugs/peptides are presented.
Conclusions. Drug development issues such as commercial viability, mechanisms involved, range of applicability, safety, scale-up and manufacture are outlined, and future research and development efforts to address these issues are discussed.
Similar content being viewed by others
REFERENCES
G. M. Eccleston. Microemulsions. In: J. Swarbrick and J. C. Boylan (eds). Encyclopedia of Pharmaceutical Technology. Marcel Dekker, New York, 1992, Vol. 9, pp. 375–421.
D. Attwood. Microemulsions. In: J. Kreuter (ed). Colloidal Drug Delivery Systems. Marcel Dekker, New York, 1994, pp. 31–71.
W. A. Ritschel. Microemulsions for improved peptide absorption from the gastrointestinal tract. Meth. Find. Exp. Clin. Pharmacol. 13: 205–220 (1993).
C. W. Pouton. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int. J. Pharm. 27: 335–348 (1985).
S. A. Charman, W. N. Charman, M. C. Rogge, T. D. Wilson, F. J. Dutko and C. W. Pouton. Self-emulsifying drug delivery systems: formulation and biopharmaceutical evaluation of an investigational lipophilic compound. Pharm. Res. 9: 87–93, (1992).
N. H. Shah, M. T. Carvajal, C. I. Patel, M. H. Infeld and A. W. Malick, Self-emulsifying drug delivery systems with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int. J. Pharm. 106: 15–23 (1994).
R. J. Ptachcinsky, R. Venkataramanan, and G. J. Burckart, Clinical pharmacokinetics of Cyclosporin. Clin. Pharmacokinet. 11: 107–132 (1986).
J.M. Kovarik, E. A. Mueller, J. B. van Bree, W. Tetzloff and K. Kutz, Reduced inter-and intraindividual variability in Cyclosporine pharmacokinetics from a microemulsion formulation. J. Pharm. Sci. 83: 444–446 (1994).
Greiner, R. W. and D. F. Evans, Spontaneous formation of a water-continuous emulsion from water-in-oil microemulsion. Langmuir 6: 1793–1796 (1990).
P. P. Constantinides and S. H. Yiv. Particle size of phase inverted water-in-oil microemulsions under different dilution and storage conditions. Int. J. Pharm. 115: 225–234 (1995).
P. P. Constantinides, J.P. Scalart, C. Lancaster, J. Marcello, G. Marks, H. Ellens and P. L. Smith. Formulation and intestinal absorption enhancement evaluation of water-in-oil microemulsions incorporating medium-chain glycerides. Pharm. Res. 11: 1385–1390 (1994).
P. P. Constantinides, C. M. Lancaster, J. Marcello, D. Chiossone, D. Orner, I. Hidalgo, P. L. Smith, A. B. Sarkahian, S. H. Yiv and A. J. Owen. Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J. Control. Rel. 34: 109–116 (1995).
E. C. Swenson and W. J. Curatolo. Intestinal permeability enhancement for proteins, peptides and other polar drugs: mechanisms and potential toxicity. Adv. Drug Deliv. Rev. 8: 39–92, (1992).
D. W. Osborne, C. A. Middleton, and R. L. Rogers. Alcoholfree microemulsions. J. Dispersion Sci. Technol. 9: 415–423 (1988).
P. P. Constantinides and J-P. Scalart. Self-emulsifying water-in-oil microemulsions in drug delivery: Formulation and physical characterization. Particul. and Process. Sci. Technol.: An Int. Journal 1(1): in press (1995).
M. Sekine, H. Terashima, K. Sasahara, K. Nishimura, R. Okada and S. Awazu. Improvement of bioavailability of poorly absorbed drugs II. Effect of medium-chain glyceride base on the intestinal absorption of cefmetazole sodium in rats and dogs. J. Pharmacobio-Dyn. 8: 286–295 (1985).
M. Sekine, K. Sasahara, R. Okada and S. Awazu. Improvement of bioavailability of poorly absorbed drugs. IV. Mechanism of the promoting effect of medium-chain glyceride on the rectal absorption of water soluble drugs. J. Pharmacobio-Dyn. 8: 645–652 (1985).
G. Beskid, J. Unowsky, C. R. Behl, J. Siebelist, J. L. Tossounian, C. M. McGarry, N. H. Shah and R. Cleeland. Enteral, oral and rectal absorption of Ceftriaxone using glyceride enhancers. Chemother. 34: 77–84 (1988).
I. Holmberg, L. Aksnes, T. Berlin, B. Lindback, J. Zemgals and B. Lindeke. Absorption of a pharmacological dose of vitamin D3 from two different lipid vehicles in man: comparison of peanut oil and a medium-chain triglyceride. Biopharm. Drug Disposition 11: 807–815 (1990).
K. Shinoda, M. Araki, A. Sadaghiani, A. Khan, and B. Lindman. Lecithin-based microemulsions: Phase behavior and microstructure. J. Phys. Chem. 95: 989–993 (1991)
N. Jezyk, W. Rubans and G. M. Grass. Permeability characteristics of various intestinal regions of rabbit, dog and monkey. Pharm. Res. 9: 1580–1586 (1993).
G. L. Amidon, H. Lennernas, V. P. Shah and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12: 413–420 (1995).
B. Latreille and P. Paquin. Evaluation of emulsion stability by centrifugation and conductivity measurements. J. Food Sci. 55: 1666–1672, (1990).
G. Ktistis. A viscosity study on oil-in-water microemulsions. Int. J. Pharm. 61: 213–218 (1990).
A. M. Cabazat, D. Langevin, and A. Pouchelon. Light scattering study of water-in-oil microemulsions. J. Colloid Interface Sci. 73: 1–11 (1980).
M. Kahlweit, R. Strey, D. Haase, H. Kunieda, T. Schmeling, B. Faulhaber, M. Borkovec, H.-F. Eicke, G. Busse, F. Eggers, T.H. Funck, H. Richmann, L. Magid, O. Soderman, P. Stilbs, J. Winkler, A. Dittrich and W. Jahn. How to study microemulsions. J. Colloid Interface Sci. 118: 436–453 (1987).
Lievens, Hildegard S. R. Pharmaceutical formulations of benzodiazepines. European Patent Application EP 0 517 412 A1, 26 May, 1992.
N. Farah, J. P. Laforet and J. Denis. Self-microemulsifying drug delivery systems for improving dissolution of drugs: in vitro/in vivo evaluation. Pharm. Res. 11: S-202 (1994).
W. A. Ritschel, G. B. Ritschel, A. Sabouni, D. Wolochuk and T. Schroeder. Study on the peroral absorption of the endekapeptide cyclosporin A. Meth. Find. Exp. Clin. Pharmacol. 11: 281–287 (1989).
W. A. Ritschel, S. Adolph, G. B. Ritschel and T. Schroeder. Improvement of peroral absorption of cyclosporin A by microemulsions. Meth. Find. Exp. Clin. Pharmacol. 12: 127–134 (1990)
P. L. Smith, D. A. Wall and G. Wilson. Drug carriers for the oral administration and transport of peptide drugs across the gastrointestinal epithelium. In: A. Rolland (ed). Pharmaceutical Particulate Carriers: Therapeutic Applications. Marcel Dekker, New York, 1993, pp. 109–166.
H. Westergaard and J. M. Dietschy. The uptake of lipids into the intestinal mucosa. In: T.E. Andreoli, J. F. Hoffman, D. D. Fanestil and S. G. Schultz, (eds). Membrane Transport Processes in Organized Systems. Plenum, New York, 1987, pp. 213–224.
D. W. Holt, E. A. Mueller, J. M. Kovarik, J. B. van Bree and K. Kutz. The pharmacokinetics of Sandimmune Neoral: A new oral formulation of cyclosporine. Transplant. Proc. 26: 2935–2939 (1994).
E. A. Mueller, J. M. Kovarik, J. B. van Bree, W. Tetzloff, J. Grevel and K. Kutz. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm. Res. 11: 301–304 (1994).
A. J. Owen, S. H. Yiv and Ani Sarkahian. Convertible microemulsion formulations. PCT Patent Application WO 92/18147, 29 October 1992.
P. P. Constantinides. Water-in-oil microemulsions. PCT Patent Application WO 93/02664, 18 February 1993
P. P. Constantinides and S. H. Yiv. Pharmaceutical emulsion compositions. PCT Patent Application WO 94/08610, 28 April 1994.
Y. W. Cho. Pharmaceutical Compositions. U.S Patent 4, 849, 227, 18 July 1989.
W. A. Ritschel, G. B. Ritschel and G. Sathyan. Insulin drug delivery systems: rectal gels. Res. Commun. Chem. Pathol. Pharmacol. 62: 103–112 (1988).
J. Samanen, F. Ali, T. Romoff, R. Calvo, E. Sorenson, J. Vasko, B. Storer, D. Berry, D. Bennett, M. Strohsacker, D. Powers, J. Stadel and A. Nichols. Development of a small RGD peptide fibrinogen receptor antagonist with potent antiaggregatory activity in vitro. Med. Chem. 34: 3114–3125 (1991).
R. A. Myers and V. J. Stella, Systemic bioavailability of penclomedine (NSC-338720) from oil-in-water emulsions administered intraduodenally to rats. Int. J. Pharm. 78: 217–226 (1992).
T. T. Kararli, T. E. Needham, M. Griffin, G. Schoenhard, L. J. Ferro and L. Alcorn, Oral delivery of a renin inhibitor compound using emulsion formulations. Pharm. Res. 9: 888–893 (1992).
Y. Yamahira, T. Noguchi, H. Takenaka and T. Maeda. Biopharmaceutical studies of lipid-containing oral dosage forms: Relationship between drug absorption and gastric emptying of lipid formulations. J. Pharmacobio Dyn. 1: 160–167 (1978).
S. T. Nielsen, S. H. Yiv, P. Dentinger, A. Dickason and A. Owen. Microemulsion-based drug delivery system: Characterization of pharmacokinetic parameters of calcein absorption in the rat. Pharm. Res. 10: S 293 (1993).
V.H.L. Lee, A. Yamamoto and U. B. Kompella. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Crit. Rev. Ther. Drug Carrier Syst. 8: 91–192 (1991).
P.-Y. Yeh, P. L. Smith and H. Ellens. Effect of medium-chain glycerides on physiological properties of rabbitt intestinal epithelium in vitro. Pharm. Res. 11: 1148–1154 (1994).
D. S. Hsieh. Understanding permeation enhancement technologies. In: D. S. Hsieh (ed). Drug permeation enhancement: Theory and applications. Marcel Dekker, New York, 1994, pp. 3–17.
E. S. Swenson, W. B. Milisen and W. Curatolo. Intestinal permeability enhancement: efficacy, acute local toxicity and reversibility. Pharm. Res. 11: 1132–1142 (1994).
N. A. Armstrong and K. C. James. Drug release from lipid-based dosage forms. II. Int. J. Pharm. 6: 195–204 (1980).
M. Ttotta, M. R. Gasco and S. Morel. Release of drugs from oil-in-water microemulsions. J. Control. Rel. 10: 237–243 (1989).
G. A. van Burskirk, V. P. Shah, D. Adair, H. M. Arbit, S. V. Dighe, M. Fawzi, T. Feldman, G. L. Flynn, M. A. Gonzalez, V. A. Gray, R. H. Guy, A. K. Herd, S. L. Hem, C. Hoiberg, R. Jerussi, A. S. Kaplan, L. J. Lesko, H. M. Malinowski, N.M. Meltzer, R. L. Nedich, D. M. Pearce, G. Peck, A. Rudman, D. Savello, J. B. Schwartz, P. Schwartz, J. P. Skelly, R. K. Vanderlaan, J. C. T. Wang, N. Weiner, D. R. Winkel and J. L. Zatz. Workshop III Report: Scale-up of liquid and semisolid disperse systems. Eur. J. Pharm. Biopharm. 40: 251–254 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Constantinides, P.P. Lipid Microemulsions for Improving Drug Dissolution and Oral Absorption: Physical and Biopharmaceutical Aspects. Pharm Res 12, 1561–1572 (1995). https://doi.org/10.1023/A:1016268311867
Issue Date:
DOI: https://doi.org/10.1023/A:1016268311867