Abstract
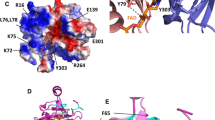
Chloroplast NADP-dependent malate dehydrogenase (NADP-MDH, EC 1.1.1.82) is inactive in the dark and activated in the light via a reduction of specific disulfides by thiol-disulfide interchange with thioredoxin, reduced by the photosynthetic electron transfer. Compared to the constitutively active NAD-dependent forms, NADP-MDH exhibits two regulatory disulfides per subunit, one located in an N-terminal extension and the other in a C-terminal extension. Convergent information gathered from biochemical, site-directed mutagenesis and structural approaches allowed to solve almost completely the activation mechanism. In the oxidized enzyme, the C-terminal extension is pulled back by the disulfide bridge toward the active-site cleft where the penultimate C-terminal glutamate interacts with one of the arginines involved in substrate binding, thus acting as an internal inhibitor obstructing the access of oxaloacetate. The N-terminal extensions are located at the subunit interface area and rigidify the overall structure of the dimer. Their reduction by reduced thioredoxin triggers a conformational change of the active site towards high-activity conformation, whereas the reduction of the C-terminal bridge expells the C-terminal end from the active site, thus opening the way for the substrate.
Similar content being viewed by others
References
Anderson LE and Avron M (1976) Light modulation of enzyme activity in chloroplasts. Generation of membrane-bound vicinal dithiols by photosynthetic electron transport. Plant Physiol 57: 209-213
Ashton AR and Hatch MD (1983) Regulation of C4 photosynthesis: regulation of activation and inactivation of NADP-malate dehydrogenase by NADP and NADPH. Arch Biochem Biophys 227: 416-425
Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Nast G, Rodgers JJM, Müller-Rober B and Scheibe R (1998) Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207: 105-114
Backhausen JE, Kitzmann C, Horton P and Scheibe R (2000) Electron acceptors in intact spinach chloroplasts act hierarchically to prevent over-reduction and competition for electrons. Photosynth Res 64: 1-13
Beaujean A, Issakidis-Bourguet E, Catterou M, Dubois F, Sangwan-Norreel B and Sangwan R (2001) Integration and expression of Sorghum C4 phosphoenolpyruvate carboxylase and chloroplastic NADP+-malate dehydrogenase separately or together in C3 potato plants. Plant Sci 160: 1199-1210
Birktoft JJ and Banaszak LJ (1983) The presence of a histidine-aspartic acid pair in the active site of 2-hydroxyacid dehydrogenases. X-ray refinement of cytoplasmic malate dehydrogenase. J Biol Chem 258: 472-482
Birktoft JJ, Rhodes G and Banaszak LJ (1989) Refined crystal structure of cytoplasmic malate dehydrogenase at 2.5 Å resolution. Biochemistry 28: 6065-6087
Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31: 341-374
Buchanan BB (1991) Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status and future development. Arch Biochem Biophys 288: 1-9
Carr P, Verger D, Ashton AR and Ollis D (1999) Chloroplast NADP-malate dehydrogenase: structural basis of light-dependent regulation of activity by thiol oxidation and reduction. Structure 7: 461-475
Chiadmi M, Navaza A, Miginiac-Maslow M, Jacquot J-P and Cherfils J (1999) Redox signalling in chloroplasts: structure of oxidized pea fructose-1,6-bisphosphate phosphatase. EMBO J 18: 6809-6815
Crétin C, Luchetta P, Joly C, Decottignies P, Lepiniec L, Gadal P, Sallantin M, Huet JC and Pernollet JC (1990) Primary structure of sorghum malate dehydrogenase (NADP) deduced from cDNA sequence. Homology with malate dehydrogenase (NAD). Eur J Biochem 192: 299-303
de Lamotte-Guéry F, Pruvost C, Minard P, Delsuc M-A, Miginiac-Maslow M, Schmitter JM, Stein M and Decottignies P (1997) Structural and functional roles of a conserved proline residue in the ?2 helix of Escherichia coli thioredoxin. Protein Engin 10: 1425-1432
Decottignies P, Schmitter J-M, Miginiac-Maslow M, Le Maréchal P, Jacquot JP and Gadal P (1988) Primary structure of the light-dependent regulatory site of corn NADP-malate dehydrogenase. J Biol Chem 263: 11780-11785
Edwards GE, Nakamoto H, Burnell JN and Hatch MD (1985) Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annu Rev Plant Physiol 36: 255-286
Faske M, Holtgrefe S, Ocheretina O, Meister M, Backhausen JE and Scheibe R (1995) Redox equilibria between the regulatory thiols of light/dark-modulated chloroplast enzymes and dithiothreitol: fine-tuning by metabolites. Biochim Biophys Acta 1247: 135-142
Faske M, Backhausen JE, Sendker M, Singer-Bayerle M, Scheibe R and von Schaewen A (1997) Transgenic tobacco plants expressing pea chloroplast NADP-MDH cDNA in sense and antisense orientation. Plant Physiol 115: 705-715
Ferentz AE and Wagner G (2000) NMR spectroscopy: a multifaceted approach to macromolecular structure. Q Rev Biophys 33: 29-65
Ferté N, Jacquot JP and Meunier JC (1986) Structural, immunological and kinetic comparisons of NADP-dependent malate dehydrogenases from spinach (C3) and corn (C4) chloroplasts. Eur J Biochem 154: 587-595
Fickensher K and Scheibe R (1988) Limited proteolysis of inactive tetrameric chloroplast NADP-malate dehydrogenase produces active dimers. Arch Biochem Biophys 260: 771-779
Fridlyand LE, Backhausen JE and Scheibe R (1998) Flux control of the malate valve in leaf cells. Arch Biochem Biophys 349: 290-298
Gallardo F, Miginiac-Maslow M, Sangwan RS, Decottignies P, Keryer E, Dubois F, Bismuth E, Galvez S, Sangwan-Norreel B, Gadal P and Crétin C (1995) Monocotyledonous C-4 NADP(+)-malate dehydrogenase is efficiently synthesized, targeted to chloroplasts and processed to an active form in transgenic plants of the C-3 dicotyledon tobacco. Planta 197: 324-332
Gomez I, Merchan F, Fernandez E and Quesada A (2002) NADP-malate dehydrogenase from Chlamydomonas: prediction of new structural determinants for redox regulation by homology modeling. Plant Mol Biol 48: 211-221
Goward CR and Nicholls DJ (1994) Malate dehydrogenase: a model for structure, evolution and catalysis. Prot Sci 3: 1883-1888
Goyer A, Decottignies P, Lemaire S, Ruelland E, Issakidis-Bourguet E, Jacquot JP and Miginiac-Maslow M (1999) The internal Cys207 of sorghum leaf NADP-malate dehydrogenase can form mixed disulphides with thioredoxin. FEBS Lett 444: 165-169
Goyer A, Decottignies P, Issakidis-Bourguet E and Miginiac-Maslow M (2001) Sites of interaction of thioredoxin with sorghum NADP-malate dehydrogenase. FEBS Lett 505: 405-408
Hall MD, Levitt DG and Banaszak LJ (1992) Crystal structure of Escherichia coli malate dehydrogenase: a complex of the apoenzyme and citrate at 1.87 Å resolution. J Mol Biol 226: 867-882
Hatch MD and Agostino A (1992) Bilevel disulfide group reduction in the activation of C4 leaf nicotinamide adenine dinucleotide phosphate-malate dehydrogenase. Plant Physiol 100: 360-366
Hirasawa M, Schürmann P, Jacquot JP, Manieri W, Jacquot P, Keryer E, Hartman F and Knaff D (1999) Oxidation-reduction properties of chloroplast thioredoxins, ferredoxin-thioredoxin reductase and thioredoxin f-regulated enzymes. Biochemistry 38: 5200-5205
Hirasawa M, Ruelland E, Schepens I, Issakidis-Bourguet E, Miginiac-Maslow M and Knaff D (2000) Oxidation-reduction properties of the regulatory disulfides of sorghum chloroplast NADP-malate dehydrogenase. Biochemistry 39: 3344-3350
Hutchison RS, Groom Q and Ort DR (2000) Differential effects of chilling-induced photooxidation on the redox regulation of photosynthetic enzymes. Biochemistry 39: 6679-6688
Issakidis E, Miginiac-Maslow M, Decottignies P, Jacquot JP, Crétin C and Gadal P (1992) Site-directed mutagenesis reveals the involvement of an additional thioredoxin-dependent regulatory site in the activation of recombinant sorghum leaf NADP-malate dehydrogenase. J Biol Chem 267: 21577-21583
Issakidis E, Saarinen M, Decottignies P, Jacquot JP, Crétin C, Gadal P and Miginiac-Maslow M (1994) Identification and characterization of the second regulatory disulfide bridge of recombinant sorghum leaf NADP-malate dehydrogenase. J Biol Chem 269: 3511-3517
Issakidis E, Lemaire M, Decottignies P, Jacquot JP and Miginiac-Maslow M (1996) Direct evidence for the different roles of the N-and C-terminal regulatory disulfides of sorghum leaf NADP-malate dehydrogenase in its activation by reduced thioredoxin. FEBS Lett 392: 121-124
Jackson RM, Sessions RB and Holbrook JJ (1992) A prediction of the three-dimensional structure of maize NADP+-dependent malate dehydrogenase which explains aspects of light-dependent regulation unique to plant enzymes. J Comput-Aided Mol Design 6: 1-18
Jacquot JP, Lancelin JM and Meyer Y (1997) Thioredoxins: structure and function in plant cells. New Phytol 136: 543-570
Johansson K, Ramaswamy S, Saarinen M, Lemaire-Chamley M, Issakidis-Bourguet E, Miginiac-Maslow M and Eklund H (1999) Structural basis for light-activation of a chloroplast enzyme. The structure of NADP-malate dehydrogenase in its oxidized form. Biochemistry 38: 4319-4326
Johnson HS and Hatch MD (1970) Properties and regulation of leaf nicotinamide-adenine-dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4 dicarboxylic acid pathway of photosynthesis. Biochem J 119: 273-280
Kampfenkel K (1993) Limited proteolysis of NADP-malate dehydrogenase from pea chloroplast by aminopeptidase-K yields monomers-evidence of proteolytic degradation of NADP-malate dehydrogenase during purification from pea. Biochim Biophys Acta 1156: 71-77
Kelly CA, Nishiyama M, Ohnishi Y, Beppu T and Birktoft JJ (1993) Determinants of protein thermostability observed in the 1.9 A crystal strucutre of malate dehydrogenase from the thermophilic bacterium Thermus flavus. Biochemistry 32: 3913-3922
Kobe B and Kemp BE (1999) Active site-directed protein regulation. Nature 402: 373-376
Krimm I, Goyer A, Issakidis-Bourguet E, Miginiac-Maslow M and Lancelin JM (1999) Direct NMR observation of the thioredoxin-mediated reduction of the chloroplast NADP-malate dehydrogenase provides a structural basis for the relief of autoinhibition. J Biol Chem 274: 34539-34542
Lemaire M, Schmitter JM, Issakidis E, Miginiac-Maslow M, Gadal P and Decottignies P (1994) Essential histidine at the active site of sorghum leaf NADP-dependent malate dehydrogenase. J Biol Chem 269: 27291-27296
Lemaire M, Issakidis E, Ruelland E, Decottignies P and Miginiac-Maslow M (1996a) An active-site cysteine of sorghum leaf NADP-malate dehydrogenase studied by site-directed mutagenesis. FEBS Lett 382: 137-140
Lemaire M, Miginiac-Maslow M and Decottignies P (1996b) The catalytic site of chloroplastic NADP-dependent malate dehydrogenase contains a His/Asp pair. Europ J Biochem 236: 947-952
Miginiac-Maslow M, Decottignies P, Jacquot JP and Gadal P (1990) Regulation of corn-leaf NADP-malate dehydrogenase light-activation by the photosynthetic electron flow. Effect of photoinhibition studied in a reconstituted system. Biochim Biophys Acta 1917: 273-279
Miginiac-Maslow M, Issakidis E, Lemaire M, Ruelland E, Jacquot JP and Decottignies P (1997) Light-dependent activation of NADP-malate dehydrogenase: a complex process. Aust J Plant Physiol 24: 529-542
Miginiac-Maslow M, Ruelland E, Schepens I, Issakidis-Bourguet E and Decottignies P (1998) The role of the C-terminal extension of sorghum NADP-malate dehydrogenase in the inhibition of its reductive activation by NADP. In: Garab G (ed) Photosynthesis: Mechanisms and Effects, Vol 5, pp 3699-3702. Kluwer Academic Publishers, Dordrecht, The Netherlands
Ocheretina O, Harnecker J, Rother T, Schmid R and Scheibe R (1993) Effects of N-terminal truncations upon chloroplast NADP-malate dehydrogenases from pea and spinach. Biochim Biophys Acta 1163: 10-16
Ocheretina O, Haferkamp I, Tellioglu H and Scheibe R (2000) Light-modulated NADP-malate dehydrogenases from mossfern and green algae: insights into evolution of the enzyme's regulation. Gene 258: 147-154
Pervushin K, Riek R, Wider G and Wüthrich K (1997) Proc Natl Acad Sci USA 94: 12366-12371
Rebeillé F and Hatch MD (1986) Regulation of NADP-malate dehydrogenase in C4 plants: Effect of varying NADPH to NADP ratios and thioredoxin redox state on enzyme activity in reconstituted systems. Arch Biochem Biophys 249: 164-170
Riessland R and Jaenicke R (1997) Determination of regulatory disulphide bonds of NADP-dependent malate dehydrogenase from Pisum sativum by site-directed mutagenesis. Biol Chem Hoppe Seyler 378: 983-988
Ruelland E, Lemaire-Chamley M, Le Maréchal P, Issakidis-Bourguet E, Djukic N and Miginiac-Maslow M (1997) An internal cysteine is involved in the thioredoxin-dependent activation of sorghum leaf NADP-dependent malate dehydrogenase. J Biol Chem 272: 19851-19857
Ruelland E, Johansson K, Decottignies P, Djukic N and Miginiac-Maslow M (1998) The auto-inhibition of sorghum NADP-malate dehydrogenase is mediated by a C-terminal negative charge. J Biol Chem 273: 33482-33488
Ruelland E and Miginiac-Maslow M (1999) Regulation of chloroplast enzyme activities by thioredoxins: activation or relief from inhibition? Trends Plant Sci 4: 136-141
Scheibe R (1984) Quantitation of the thiol groups involved in the reductive activation of NADP-malate dehydrogenase. Biochim Biophys Acta 788: 241-247
Scheibe R (1987) NADP-malate dehydrogenase in C3 plants: regulation and role of a light-activated enzyme. Physiol Plant 71: 393-400
Scheibe R and Jacquot JP (1983) NADP regulates the light-activation of NADP-dependent malate dehydrogenase. Planta 157: 548-553
Scheibe R, Geissler A and Rother T (1993) Analysis of biophysical differences between oxidized and reduced chloroplast NADP-malate dehydrogenase. Arch Biochem Biophys 300: 635-640
Schepens I, Decottignies P, Ruelland E, Johansson K and Miginiac-Maslow M (2000a) The dimer contact area of sorghum NADP-malate dehydrogenase: role of aspartate 101 in dimer stability and catalytic activity. FEBS Lett 471: 240-244
Schepens I, Johansson K, Decottignies P, Gillibert M, Hirasawa M, Knaff D and Miginiac-Maslow M (2000b) Inhibition of the thioredoxin-dependent activation of the NADP-malate dehydrogenase and cofactor specificity. J Biol Chem 275: 20996-21001
Schepens I, Ruelland E, Miginiac-Maslow M, Le Maréchal P and Decottignies P (2000c) The role of the active-site arginines of sorghum NADP-malate dehydrogenase in thioredox-independent activation and activity. J Biol Chem 275: 35792-35798
Schürmann P and Jacquot JP (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Mol Biol 51: 371-400
Schürmann P, Balmer Y, Stritt-Etter AL, Hirasawa M, Knaff DB, Miginiac-Maslow M and Jacquot JP (2001) Effects of mutations of the cysteines in the regulatory loop on structure and activity of chloroplast fructose-1,6-bisphosphatase (FBPase) PS2001 Proceedings: 12th International Photosynthesis Congress, S20-023. CSIRO Publishing, Melbourne, Australia
Trevanion SJ, Furbank RT and Ashton AR (1997) NADP-malate dehydrogenase in the C4 plant Flaveria bidentis: cosense suppression of activity in mesophyll and bundle sheath cells and consequences for photosynthesis. Plant Physiol 113: 1153-1165
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miginiac-Maslow, M., Lancelin, JM. Intrasteric inhibition in redox signalling: light activation of NADP-malate dehydrogenase. Photosynthesis Research 72, 1–12 (2002). https://doi.org/10.1023/A:1016099228450
Issue Date:
DOI: https://doi.org/10.1023/A:1016099228450