Abstract
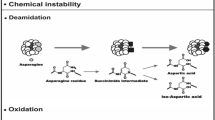
Recombinant DNA technology has now made it possible to produce proteins for pharmaceutical applications. Consequently, proteins produced via biotechnology now comprise a significant portion of the drugs currently under development. Isolation, purification, formulation, and delivery of proteins represent significant challenges to pharmaceutical scientists, as proteins possess unique chemical and physical properties. These properties pose difficult stability problems. A summary of both chemical and physical decomposition pathways for proteins is given. Chemical instability can include proteolysis, deamidation, oxidation, racemization, and β-elimination. Physical instability refers to processes such as aggregation, precipitation, denaturation, and adsorption to surfaces. Current methodology to stabilize proteins is presented, including additives, excipients, chemical modification, and the use of site-directed mutagenesis to produce a more stable protein species.
Similar content being viewed by others
REFERENCES
D. Blohm, C. Bollschweiler, and H. Hillen. Angew. Chem. Int. Ed. Engl. 27:207–225 (1988).
L. P. Gage. Am. J. Pharm. Ed. 50:368–370 (1986).
H. H. Tallan and W. H. Stein. J. Biol. Chem. 200:507–514 (1953).
U. J. Lewis and E. V. Cheever. J. Biol. Chem. 240:247–252 (1965).
U. J. Lewis, E. V. Cheever, and W. C. Hopkins. Biochim. Biophys. Acta 214:498–508 (1970).
G. W. Becker, P. M. Tackitt, W. W. Bromer, D. S. Lefeber, and R. M. Riggin. Biotech. Appl. Biochem. 10:326–337 (1988).
S. A. Berson and R. S. Yalow. Diabetes 15:875–879 (1966).
B. V. Fisher and P. B. Porter. J. Pharm. Pharmacol. 33:203–206 (1981).
F. S. M. Van Kleef, W. W. de Jong, and H. J. Hoenders. Nature 258:264–266 (1975).
T. Flatmark and K. Sletten. J. Biol. Chem. 243:1623–1629 (1968).
J. O. Minta and R. H. Painter. Immunochemistry 9:821–832 (1972).
R. P. Diaugustine, B. W. Gibson, W. Aberth, M. Kelly, C. M. Ferrua, Y. Tomooka, C. F. Brown, and M. Walker. Anal. Biochem. 165:420–429 (1987).
M. F. Perutz and J. H. Fogg. J. Mol. Biol. 138:669–670 (1980).
K. U. Yuksel and R. W. Gracy. Arch. Biochem. Biophys. 248:452–459 (1986).
P. M. Yuan, J. M. Talent, and R. W. Gray. Mech. Age. Dev. 17:151–162 (1981).
H. Maeda and K. Kuromizu. J. Biochem. (Tokyo) 81:25–35 (1977).
L. Graf, G. Cseh, I. Nagy, and M. Kurcz. Acta Biochim. Biophys. Acad. Sci. Hung. 5:299–303 (1970).
T. J. McDonald, H. Jornvall, K. Tatemoto, and V. Mutt. FEBS Lett. 156:349–356 (1983).
L. Graf, S. Bajusz, A. Patthy, E. Barat, and G. Cseh. Acta Biochim. Biophys. Acad. Sci. Hung. 6:415–418 (1971).
N. P. Bhatt, K. Patel, and R. T. Borchardt. Pharm. Res. 5:S72 (1988).
A. B. Robinson and C. J. Rudd. In B. L. Horecker and E. R. Stadtman (eds.), Current Topics in Cellular Regulations, Vol. 8, Academic Press, New York, 1974, pp. 247–295.
E. Sondheimer and R. W. Holley. J. Am. Chem. Soc. 76:2467–2470 (1954).
E. E. Haley, B. J. Coreoran, F. E. Dorer, and D. L. Buchanan. Biochemistry 5:3229–3235 (1966).
A. R. Battersby and J. C. Robinson. J. Chem. Soc. 259–269 (1955).
P. Bornstein and G. Balian. Methods. Enzymol. 47:132–145 (1977).
T. Geiger and S. Clarke. J. Biol. Chem. 262:785–794 (1987).
Y. C. Meinwald, E. R. Stimson, and H. A. Scheraga. Int. J. Peptide Protein Res. 28:79–84 (1986).
D. W. Aswad. J. Biol. Chem. 259:10714–10721 (1984).
B. A. Johnson and D. W. Aswad. Biochemistry 24:2581–2586 (1985).
P. Bornstein. Biochemistry 9:2408–2421 (1970).
A. Di Donato, P. Galletti, and G. D'Alessio. Biochemistry 25:8361–8368 (1986).
P. Galletti, A. Ciardiello, D. Ingrosso, A. Di Donato, and G. D'Alessio. Biochemistry 27:1752–1757 (1988).
S. Lou, C. Liao, J. F. McClelland, and D. J. Graves. Int. J. Peptide Protein Res. 29:728–733 (1987).
K. Patel and R. T. Borchardt. Pharm. Res. 5:S72 (1988).
K. Patel, C. Oliyai, R. T. Borchardt, and M. C. Manning. J. Cell. Biochem. 13A:88 (1989).
S. A. Bernhard, A. Berger, H. J. Carter, E. Katchalski, M. Sela, and Y. Shalitin. J. Am. Chem. Soc. 84:2421–2434 (1962).
Y. Shalitin and S. A. Bernhard. J. Am. Chem. Soc. 88:4711–4721 (1966).
M. A. Ondetti, A. Deer, J. T. Sheeham, J. Pluscec, and O. Kocy. Biochemistry 7:4069–4075 (1968).
M. Bodanszky and J. Z. Kwei. Int. J. Peptide Protein Res. 12:69–74 (1978).
S. A. Bernhard. Ann. N.Y. Acad. Sci. 421:28–40 (1983).
J. K. Blodgett, G. M. Loudon, and K. D. Collins. J. Am. Chem. Soc. 107:4305–4313 (1985).
S. Capasso, C. A. Mattia, L. Mazzarella, and A. Zagari. Int. J. Peptide Protein Res. 23:248–255 (1984).
A. J. Alder, G. D. Fasman, and E. R. Blout. J. Am. Chem. Soc. 85:90–97 (1963).
G. Perseo, R. Furino, M. Galantino, B. Gioia, V. Malatesta, and R. D. Castiglione. Int. J. Peptide Protein Res. 27:51–60 (1986).
M. Bodanszky and S. Natarajan. J. Org. Chem. 40:2495–2499 (1975).
A. B. Robinson, J. W. Scotchler, and J. H. McKerow. J. Am. Chem. Soc. 95:8156–8159 (1973).
J. W. Scotchler and A. B. Robinson. Anal. Biochem. 59:319–322 (1974).
A. B. Robinson, J. H. McKerrow, and P. Cary. Proc. Natl. Acad. Sci. USA 66:753–757 (1970).
A. B. Robinson, J. H. McKerrow, and M. Legaz. Int. J. Peptide Protein Res. 6:31–35 (1974).
T. Flatmark. Acta Chem. Scand. 20:1487–1496 (1966).
S. Clarke. Int. J. Peptide Protein Res. 30:808–821 (1987).
P. Bornstein and G. Balian. J. Biol. Chem. 245:4854–4856 (1970).
A. A. Kossiakoff. Science 240:191–194 (1988).
R. Lura and V. Schirch. Biochemistry 27:7671–7677 (1988).
M. Bodanszky, J. C. Tolle, S. S. Deshmane, and A. Bodanszky. Int. J. Peptide Protein Res. 12:57–68 (1978).
B. N. Manjula, A. S. Achary, and P. J. Vithayathil. Int. J. Peptide Protein Res. 8:275–282 (1976).
S. Charache, J. Fox, P. McCurdy, H. Kazazian, Jr., and R. Winslow. J. Clin. Invest. 59:652–658 (1977).
U. J. Lewis, R. N. P. Singh, L. F. Bonewald, and B. K. Seavey. J. Biol. Chem. 256:11645–11650 (1981).
T. J. Ahern and A. M. Klibanov. Science 228:1280–1284 (1985).
S. E. Zale and A. M. Klibanov. Biochemistry 25:5432–5444 (1986).
C. F. Midelfort and A. H. Mehler. Proc. Natl. Acad. Sci. USA 69:1816–1819 (1972).
L. Graf, G. Hajos, A. Patthy, and G. Cseh. Horm. Metab. Res. 5:142–143 (1973).
Y. P. Venkatesh and P. J. Vithayathil. Int. J. Peptide Protein Res. 25:27–32 (1985).
Y. P. Venkatesh and P. J. Vithayathil. Int. J. Peptide Protein Res. 23:494–505 (1984).
G. R. Marshall and R. B. Merrifield. Biochemistry 4:2394–2401 (1965).
C. C. Yang and R. B. Merrifield. J. Org. Chem. 41:1032–1041 (1976).
E. D. Murray, Jr., and S. Clarke. J. Biol. Chem. 259:10722–10732 (1984).
K. Bedii Oray, U. Yuksel, and R. W. Gracy. J. Chromatogr. 265:126–130 (1983).
T. Baba, H. Sugiyama, and S. Seto. Chem. Pharm. Bull. 21:207–209 (1973).
P. A. Khairallah, F. M. Bumpus, J. H. Page, and R. R. Smeby. Science 140:672–674 (1963).
C. Secchi, P. A. Biondi, A. Negri, R. Borroni, and S. Ronchi. Int. J. Peptide Protein Res. 28:298–306 (1986).
S. Clarke. In R. T. Borchardt, C. R. Creveling, and P. M. Ueland (eds.), Biological Methylation and Drug Design, Humana Press, Clifton, N.J., 1985, pp. 3–14.
B. A. Johnson, J. M. Shirokawa, and D. W. Aswad. Arch. Biochem. Biophys. 268:276–286 (1989).
H. B. F. Dixon, S. Moore, M. P. Stack-Dunne, and F. G. Young. Nature 168:1044–1045 (1951).
F. A. Kuehl, Jr., M. A. P. Meisinger, N. G. Brink, and K. Folkers. J. Am. Chem. Soc. 75:1955–1959 (1953).
H. Rasmussen and L. C. Craig. Recent Prog. Horm. Res. 18:269–295 (1962).
W. Vale, J. Spiess, C. Rivier, and J. Rivier. Science 213:1394–1397 (1981).
K. Norris, J. Halstrom, and K. Brunfeldt. Acta Chem. Scand. 25:945–954 (1971).
M. Coltrera, M. Rosenblatt, and J. T. Potts, Jr. Biochemistry 19:4380–4385 (1980).
G. E. Means and R. E. Feeney. In Chemical Modifications of Proteins, Holden-Day, New York, 1971, pp. 162–165.
N. Brot and H. Weissbach. Trends Biochem. Sci. 7:137–139 (1982).
Y. Shechter, Y. Burstein, and A. Patchornik. Biochemistry 14:4497–4503 (1975).
Y. Shechter. J. Biol. Chem. 261:66–70 (1986).
M. L. Dedman, T. H. Farmer, and C. J. O. R. Morris. Biochem. J. 78:348–352 (1961).
H. B. F. Dixon. Biochim. Biophys. Acta 19:392–394 (1956).
A. H. Tashjian, D. A. Ontjes, and P. L. Munson. Biochemistry 3:1175–1182 (1964).
J. S. Morley, H. J. Tracey, and R. A. Gregory. Nature 207:1356–1359 (1965).
V. B. Riniker, R. Neher, R. Maier, F. W. Kahnt, P. G. H. Byfield, T. V. Gudmundsson, L. Galante, and I. MacIntyre. Helv. Chem. Acta 51:1738–1742 (1968).
P. Caldwell, D. C. Luk, H. Weissbach, and N. Brot. Proc. Natl. Acad. Sci. USA 75:5349–5352 (1978).
H. Carp and A. Janoff. Am. Rev. Resp. Dis. 118:617–621 (1978).
W. R. Abrams, A. Eliraz, P. Kimbel, and G. Weinbaum. Exp. Lung Res. 1:211–223 (1980).
C. E. Stauffer and D. Etson. J. Biol. Chem. 244:5333–5338 (1969).
W. J. Ray, Jr., and D. E. Koshland, Jr. J. Biol. Chem. 237:2493–2505 (1962).
H. Schachter and G. H. Dixon. J. Biol. Chem. 239:813–829 (1964).
V. Holeysovsky and M. Lazdunski. Biochim. Biophys. Acta 154:457–467 (1968).
U. W. Kenkare and F. M. Richards. J. Biol. Chem. 241:3197–3206 (1966).
G. Jori, G. Galiazzo, A. Marzotto, and E. Scoffone. Biochim. Biophys. Acta 154:1–9 (1968).
G. Jori, G. Galiazzo, A. Marzotto, and E. Scoffone. J. Biol. Chem. 243:4272–4278 (1968).
N. P. Neumann, S. Moore, and W. H. Stein. Biochemistry 1:68–75 (1962).
H. Schachter, K. A. Halliday, and G. H. Dixon. J. Biol. Chem. 238:PC3134–PC3136 (1963).
B. Kassell. Biochemistry 3:152–155 (1964).
W. F. Heath and R. B. Merrifield. Proc. Natl. Acad. Sci. USA 83:6367–6371 (1986).
C. George-Nascimento, A. Gyenes, S. M. Halloran, J. Merryweather, P. Valenzuela, K. S. Steimer, F. R. Masiarz, and A. Randolph. Biochemistry 27:797–802 (1988).
S. A. Coolican, B. N. Jones, R. D. England, K. C. Flanders, J. D. Condit, and R. S. Gurd. Biochemistry 21:4974–4981 (1982).
L. C. Teh, L. J. Murphy, N. L. Huq, A. S. Surus, H. G. Friesen, L. Lazarus, and G. E. Chapman. J. Biol. Chem. 262:6472–6477 (1987).
H. G. Gundlach, S. Moore, and W. H. Stein. J. Biol. Chem. 234:1761–1764 (1959).
E. Gross and B. Witkop. J. Am. Chem. Soc. 83:1510–1511 (1961).
H. B. F. Dixon and M. P. Stack-Dunne. Biochem. J. 61:483–495 (1955).
T. B. Lo, J. S. Dixon, and C. H. Li. Biochim. Biophys. Acta 53:584–586 (1961).
H. P. J. Bennett, A. M. Hudson, C. McMartin, and G. E. Purdon. Biochem. J. 168:9–13 (1977).
D. C. Shaw and C. E. West. J. Chromatogr. 200:185–188 (1980).
P. I. Storring and R. J. Tiplady. Anal. Biochem. 141:43–54 (1984).
P. C. Jocelyn. Biochemistry of the SH Groups: The Occurrence, Chemical Properties, Metabolism, and Biological Function of Thiols and Disulfides, Academic Press, New York, 1972.
C. Little and P. J. O'Brien. Arch. Biochem. Biophys. 122:406–410 (1967).
H. Lamfrom and S. O. Nielson. J. Am. Chem. Soc. 79:1966–1970 (1957).
D. Cavallini, C. DeMarco, and S. Dupre. Arch. Biochem. Biophys. 124:18–26 (1968).
E. S. G. Barron, Z. B. Miller, and G. Kalnitsky. Biochem. J. 41:62–68 (1947).
C. G. Overberger and J. J. Ferraro. J. Org. Chem. 27:3539–3545 (1962).
L. Philipson. Biochim. Biophys. Acta 56:375–377 (1962).
S. J. Tomazic and A. M. Klibanov. J. Biol. Chem. 263:3086–3091 (1988).
Y. M. Torchinskii. In Sulfhydryl and Disulfide Groups of Proteins, Consultants Bureau, New York, 1974, pp. 99–124.
L. A. Æ. Sluyterman. Biochim. Biophys. Acta 60:557–561 (1962).
B. R. DasGupta and D. A. Boroff. Biochim. Biophys. Acta 97:157–159 (1965).
L. Weil. Arch. Biochem. Biophys. 110:57–68 (1965).
J. D. Spikes and R. Straight. Annu. Rev. Phys. Chem. 18:49–436 (1967).
C. S. Foote. Science 162:963 (1968).
W. J. Ray. Methods Enzymol. 11:490–497 (1967).
P. Hoffee, C. Y. Lai, E. L. Pugh, and B. L. Horecker. Proc. Natl. Acad. Sci. USA 57:107–113 (1967).
M. Martinez-Carrion, R. Kuczenski, D. C. Tiemeier, and D. L. Peterson. J. Biol. Chem. 245:799–805 (1970).
M. Martinez-Carrion, C. Turano, F. Riva, and P. Fasella. J. Biol. Chem. 242:1426–1430 (1967).
M. Nakatani. J. Biochem. (Tokyo) 48:633–639 (1960).
R. D. Hill and R. R. Laing. Biochim. Biophys. Acta 99:352–359 (1965).
J. Schulz. Methods Enzymol. 11:255–263 (1967).
A. Light. Methods Enzymol. 11:417–420 (1967).
A. S. Inglis. Methods Enzymol. 91:324–332 (1983).
D. Piszkiewicz, M. Landon, and E. L. Smith. Biochem. Biophys. Res. Commun. 40:1173–1178 (1970).
K. Poulsen, K. J. Kevin, and E. Haber. Proc. Natl. Acad. Sci. USA 69:2495–2499 (1972).
F. Marcus. Int. J. Peptide Protein Res. 25:542–546 (1985).
D. L. Swallow and E. P. Abraham. Biochem. J. 70:364–373 (1958).
M. A. Naughton, F. Sanger, B. S. Hartley, and D. C. Shaw. Biochem. J. 77:149–163 (1960).
M. Bodanszky, G. F. Sigler, and A. Bodanszky. J. Am. Chem. Soc. 95:2352–2357 (1973).
M. Bodanszky, M. A. Ondetti, S. D. Levine, and N. J. Williams. J. Am. Chem. Soc. 89:6753–6757 (1967).
K. K. Han, C. Richard, and G. Biserte. Int. J. Biochem. 15:875–884 (1983).
P. Desnuelle and A. Casal. Biochim. Biophys. Acta 2:64–75 (1948).
U. K. Laemmli. Nature 227:680–685 (1970).
J. B. Fleischman. Immunochemistry 10:401–407 (1973).
V. Braun and W. A. Schroeder. Arch. Biochem. Biophys. 118:241–252 (1967).
W. R. Gray. Methods Enzymol. 25:121–138 (1972).
J. L. Meuth. Biochemistry 21:3750–3757 (1982).
P. Edman and G. Begg. Eur. J. Biochem. 1:80–91 (1967).
K. A. Walsh, L. H. Ericsson, D. C. Parmelee, and K. Titani. Annu. Rev. Biochem. 50:261–284 (1981).
D. Kahne and W. C. Still. J. Am. Chem. Soc. 110:7529–7534 (1988).
R. Cecil and J. R. McPhee. Adv. Protein Chem. 14:255–389 (1959).
V. L. Lumper and H. Zahn. Adv. Enzymol. Relat. Areas Mol. Biol. 27:199–238 (1965).
D. B. Volkin and A. M. Klibanov. J. Biol. Chem. 262:2945–2950 (1987).
R. E. Benesch and R. Benesch. J. Am. Chem. Soc. 80:1666–1669 (1958).
A. P. Ryle and F. Sanger. Biochem. J. 60:535–540 (1955).
Y. M. Torchinsky. In Sulfur in Proteins, Pergamon Press, New York, 1981, pp. 82–86.
E. Haber and C. B. Anfinsen. J. Biol. Chem. 237:1839–1844 (1962).
A. Galat, T. E. Creighton, R. C. Lord, and E. R. Blout. Biochemistry 20:594–601 (1981).
A. Neuberger. Adv. Protein Chem. 4:297–383 (1948).
G. G. Smith and B. S. Desol. Science 207:765–767 (1980).
M. Friedman and P. M. Masters. J. Food Sci. 47:760 (1982).
P. J. M. van den Oestelaar and H. J. Hoenders. Adv. Exp. Med. Biol. 231:261–267 (1988).
T. M. Florence. Biochem. J. 189:507–520 (1980).
J. R. Whitaker and R. E. Feeney. CRC Crit. Rev. Food Sci. Nutr. 19:173–212 (1983).
L. C. Sen, E. Gonzalez-Flores, R. E. Feeney, and J. R. Whitaker. J. Agr. Food Chem. 25:632–638 (1977).
H. S. Lee, D. T. Osuga, A. S. Nashef, A. I. Ahmed, J. R. Whitaker, and R. E. Feeney. J. Agr. Food Chem. 25:1153–1158 (1977).
A. S. Nashef, D. T. Osuga, H. S. Lee, A. I. Ahmed, J. R. Whitaker, and R. E. Feeney. J. Agr. Food Chem. 25:245–251 (1977).
T. E. Creighton. Proteins, W. H. Freeman, New York, 1983.
C. Ghelis and J. Yon. Protein Folding, Academic Press, New York, 1982.
W. Kauzmann. Adv. Protein Chem. 14:1–63 (1959).
P. L. Privalov. Adv. Protein Chem. 33:167 (1979).
K. Dill. Biochemistry 24:1501–1509 (1985).
Y. R. Hsu and T. Arakawa. Biochemistry 24:7959–7963 (1985).
T. Arakawa and W. C. Kenney. Int. J. Peptide Protein Res. 31:468–473 (1988).
T. Arakawa, T. Boone, J. M. Davis, and W. C. Kenney. Biochemistry 25:8274–8277 (1986).
T. Arakawa, Y.-R. Hsu, and D. A. Yphantis. Biochemistry 26:5428–5432 (1987).
W. W. Fish, A. Danielsson, K. Nordling, S. H. Miller, C. F. Lam, and I. Björk. Biochemistry 24:1510–1517 (1985).
D. N. Brems, S. M. Plaisted, E. W. Kauffman, and H. A. Havel. Biochemistry 25:6539–6543 (1986).
T. F. Holzman, D. N. Brems, and J. J. Dougherty, Jr. Biochemistry 25:6907–6917 (1986).
D. N. Brems, S. M. Plaisted, H. A. Havel, and C.-S. C. Tomich. Proc. Natl. Acad. Sci. USA 85:3367–3371 (1988).
D. N. Brems. Biochemistry 27:4541–4546 (1988).
Y. Goto and A. L. Fink. Biochemistry 28:945–952 (1989).
J. Baum, C. M. Dobson, P. A. Evans, and C. Hanley. Biochemistry 28:7–13 (1989).
D. N. Brems, S. M. Plaisted, J. J. Dougerty, Jr., and T. F. Holzman. J. Biol. Chem. 262:2590–2594 (1987).
D. N. Brems, S. M. Plaisted, H. A. Havel, E. W. Kauffman, J. D. Stodola, L. C. Eaton, and R. C. White. Biochemistry 24:7662–7668 (1985).
H. A. Havel, E. W. Kauffman, S. M. Plaisted, and D. N. Brems. Biochemistry 25:6533–6538 (1986).
W. Pfeil. Mol. Cell. Biochem. 40:2–38 (1981).
M. P. Tombs. J. Appl. Biochem. 7:3–24 (1985).
W. J. Becktel and J. A. Schellman. Biopolymers 26:1859–1877 (1987).
C. Tanford. Adv. Protein Chem. 23:121–282 (1968).
J. A. Schellman. Annu. Rev. Biophys. Biophys. Chem. 16:115–137 (1987).
C. N. Pace. CRC Crit. Rev. Biochem. 3:1–43 (1975).
H. Neurath, J. P. Greenstein, F. W. Putnam, and J. O. Erickson. Chem. Rev. 34:157–265 (1944).
J. M. Thornton. J. Mol. Biol. 151:261–287 (1981).
T. J. Ahern and A. M. Klibanov. Meth. Biochem. Anal. 33:91–127 (1985).
C. B. Anfinsen and H. A. Scheraga. Adv. Protein Chem. 29:205–300 (1975).
T. E. Creighton. Prog. Biophys. Mol. Biol. 33:231–297 (1978).
P. L. Privalov. Adv. Protein Chem. 35:1 (1982).
J. F. Brandts. In S. N. Timasheff and G. D. Fasman (eds.), Biological Macromolecules Series, Vol. 2, Structure and Stability of Biological Macromolecules, Marcel Dekker, New York, 1967, p. 213.
R. Wetzel, L. J. Perry, W. A. Baase, and W. J. Becktel. Proc. Natl. Acad. Sci. USA 85:401–405 (1988).
W. J. Becktel and W. A. Baase. Biopolymers 26:619–623 (1987).
J. Novotny, A. A. Rashin, and R. E. Bruccoleri. Proteins: Structure, Function, Genetics 4:19–30 (1988).
M. H. Zehfus and G. D. Rose. Biochemistry 25:5759–5765 (1986).
S. H. Bryant and L. M. Anzel. Int. J. Peptide Protein Res. 29:46–52 (1986).
A. M. Klibanov. Adv. Appl. Microbiol. 29:1–28 (1983).
R. Lumry and H. Eyring. J. Phys. Chem. 58:110–120 (1954).
T. Arakawa, N. K. Alton, and Y.-R. Hsu. J. Biol. Chem. 260:14435–14439 (1985).
D. A. Yphantis and T. Arakawa. Biochemistry 26:5422–5427 (1987).
T. Hoshino, Y. Mikura, H. Shimidzu, J. Kawai, and H. Toguchi. Biochim. Biophys. Acta 916:245–250 (1987).
T. A. Horbett. ACS Adv. Chem. Ser. 199:233–244 (1982).
T. A. Horbett. ACS Symp. Ser. 343:239–260 (1987).
J. R. Brennan, S. S. P. Gebhart, and W. G. Blackard. Diabetes 34:353–359 (1985).
D. E. James, A. B. Jenkins, E. W. Kraegen, and D. J. Chisholm. Diabetologia 21:554–557 (1981).
W. D. Lougheed, H. Woulfe-Flanagan, J. R. Clement, and A. M. Albisser. Diabetologia 19:1–9 (1980).
L. Peterson, J. Caldwell, and J. Hoffman. Diabetes 25:72–74 (1976).
M. V. Sefton. ACS Adv. Chem. Ser. 199:511–522 (1982).
A. S. Chawla, I. Hinberg, P. Blais, and D. Johnson. Diabetes 34:420–424 (1985).
G. K. Iwamoto, R. A. Van Wagenen, and J. D. Andrade. J. Colloid Interface Sci. 86:581–585 (1982).
E. H. Massey and T. A. Sheliga. Pharm. Res. 5:S34 (1988).
W. D. Lougheed, A. M. Albisser, H. M. Martindale, J. C. Chow, and J. R. Clement. Diabetes 32:424–432 (1983).
S. Sato, C. D. Ebert, and S. W. Kim. J. Pharm. Sci. 72:228–232 (1983).
Z. J. Twardowski, K. D. Nolph, T. J. McGary, and H. L. Moore. Am. J. Hosp. Pharm. 40:583–586 (1983).
Z. J. Twardowski, K. D. Nolph, T. J. McGary, H. L. Moore, P. Collin, R. K. Ausman, and W. S. Slimack. Am. J. Hosp. Pharm. 40:575–579 (1983).
Z. J. Twardowski, K. D. Nolph, T. J. McGary, and H. L. Moore. Am. J. Hosp. Pharm. 40:579–581 (1983).
M. L. Anson. In C. L. A. Schmidt (ed.), The Chemistry of the Amino Acids and Proteins, Charles Thomas, Springfield, Ill., 1938, pp. 407–428.
A. E. Mirsky and L. Pauling. Proc. Natl. Acad. Sci. USA 22:439–447 (1936).
J. K. Krueger, M. H. Kulke, C. Schutt, and J. Stock. BioPharm. Mar.:41–45 (1989).
F. A. O. Marston. Biochem. J. 240:1–12 (1986).
J. M. Schoemaker, A. H. Brasnett, and F. A. O. Marston. EMBO J. 4:775–780 (1985).
D. L. Hartley and J. F. Kane. Biochem. Soc. Trans. 16:101–102 (1988).
D. C. Williams, R. M. Van Frank, W. L. Muth, and J. P. Burnett. Science 215:687 (1982).
J. King. Bio/technology 4:297–303 (1986).
M. Gribskov and R. R. Burgess. Gene 26:109–118 (1983).
F. A. O. Marston, P. A. Lowe, M. T. Doel, J. M. Schoemaker, S. White, and S. Angal. Bio/technology 2:800–804 (1984).
S. Cabilly, A. D. Riggs, H. Pande, J. E. Shirely, W. E. Holmes, M. Rey, L. J. Perry, R. Wetzel, and H. L. Heynecker. Proc. Natl. Acad. Sci. USA 81:3273–3277 (1984).
M. E. Winkler, M. Blabel, G. L. Bennett, W. Holmes, and G. A. Vehar. Bio/technology 3:990–1000 (1985).
H. J. George, J. J. L'Italien, W. P. Pilancinski, D. L. Glassman, and R. A. Krzyzek. DNA 4:273–281 (1985).
R. C. Fahey, J. S. Hunt, and G. C. Winham. J. Mol. Evol. 10:155–160 (1977).
T. Arakawa and S. N. Timasheff. Biochemistry 23:5912–5923 (1984).
T. Arakawa and S. N. Timasheff. Biochemistry 21:6545–6552 (1982).
F. Ahmad and C. C. Bigelow. J. Protein Chem. 5:355–367 (1986).
R. Bhat and J. C. Ahluwalia. Int. J. Peptide Protein Res. 30:145–152 (1985).
F. Ahmad. Can J. Biochem. Cell Biol. 63:1058–1063 (1985).
R. Almog. Biophys. Chem. 17:111–118 (1983).
E. Stellwagen and J. Babul. Biochemistry 14:5135–5140 (1975).
L. O. Narhi, M. K. Zukowski, and T. Arakawa. Biophys. J. 55:23a (1989).
M. W. Pantoliano, M. Whitlow, J. F. Wood, M. L. Rollence, B. C. Finzel, G. L. Gilliland, T. L. Poulos, and P. N. Bryant. Biochemistry 27:8311–8317 (1988).
A. J. Russell and A. R. Fersht. Nature 328:496–500 (1987).
C. N. Pace and G. R. Grimsley. Biochemistry 27:3242–3246 (1988).
F. W. Dahlquist, J. W. Long, and W. L. Bigbee. Biochemistry 15:1103–1111 (1976).
G. Voordouw, C. Milo, and R. S. Roche. Biochemistry 15:3716–3724 (1976).
V. V. Filimonov, W. Pfeil, T. N. Tsalkova, and P. L. Privalov. Biophys. Chem. 8:117–122 (1978).
H. Schulz. FEBS Lett. 78:303–308 (1977).
J. F. Chlebowski, S. Mabrey, and M. C. Falk. J. Biol. Chem. 254:5745–5753 (1979).
J. F. Chlebowski and S. Mabrey. J. Biol. Chem. 252:7042–7050 (1977).
S. Linse, P. Brodin, C. Johansson, E. Thulin, T. Grundström, and S. Forsén. Nature 335:651–653 (1988).
E. Stellwagen and H. Wilgus. In S. M. Friedman (ed.), Biochemistry of Thermophily, Academic Press, New York, 1978, pp. 228–232.
J. A. Roe, A. Butler, D. M. Scholler, J. S. Valentine, L. Marky, and K. J. Breslauer. Biochemistry 27:950–958 (1988).
Y. Hiroka, T. Segawa, K. Kawajima, S. Sugai, and N. Murai. Biochem. Biophys. Res. Commun. 95:1098–1104 (1980).
M. Mitani, Y. Harushima, K. Kawajima, M. Ikeguchi, and S. Sugai. J. Biol. Chem. 261:8824–8829 (1986).
J. Desmet, I. Hanssens, and F. van Cauwelaert. Biochim. Biophys. Acta 912:211–219 (1987).
R. Palmieri, R. W.-K. Lee, and M. F. Dunn. Biochemistry 27:3387–3397 (1988).
K. Gekko and S. N. Timasheff. Biochemistry 20:4667–4676 (1981).
K. Gekko and S. N. Timasheff. Biochemistry 20:4677–4686 (1981).
J. C. Lee and S. N. Timasheff. J. Biol. Chem. 256:7193–7201 (1981).
J. C. Lee and S. N. Timasheff. Biochemistry 13:257–265 (1974).
J. C. Lee and S. N. Timasheff. Biochemistry 14:5183–5187 (1975).
S. N. Timasheff, J. C. Lee, E. P. Pittz, and N. Tweedy. J. Colloid Interface Sci. 55:658–663 (1976).
J. C. Lee and S. N. Timasheff. Biochemistry 16:1754–1764 (1977).
I. D. Kuntz, Jr., and W. Kauzmann. Adv. Protein Chem. 28:239–345 (1974).
V. Prakesh, P. K. Nandi, and B. Jirgensons. Int. J. Peptide Protein Res. 15:305–313 (1980).
B. Jirgensons. J. Protein Chem. 1:71 (1982).
B. Jirgensons. Macromol. Chem. Rapid Commun. 2:213–217 (1981).
F. F. Shih and A. D. Kalmar. J. Agr. Food Chem. 35:672–675 (1987).
K. Takeda, K. Sasa, M. Nagao, and P. P. Batra. Biochim. Biophys. Acta 957:340–344 (1988).
K. Fukushima, Y. Murata, N. Nishikido, G. Sugihara, and M. Tanaka. Bull. Chem. Soc. Jap. 54:3122–3127 (1981).
J. L. Bohnert and T. A. Horbett. J. Colloid Interface Sci. 111:363–377 (1986).
J. Piatigorsky, J. Horwitz, and R. T. Simpson. Biochim. Biophys. Acta 490:279–289 (1977).
S. Tandon and P. M. Horowitz. J. Biol. Chem. 262:4486–4491 (1987).
P. S. Banerjee and W. A. Ritschel. J. Pharm. Sci. 76:S48 (1987).
A. L. Daugherty, H. D. Liggitt, J. G. McCabe, J. A. Moore, and J. S. Patton. Int. J. Pharm. 45:197–206 (1988).
D. Shortle. J. Biol. Chem. 264:5315–5318 (1989).
E. Querol and A. Padilla. Enzyme Microbial Technol. 9:238–244 (1987).
M. H. Hecht, J. M. Sturtevant, and R. T. Sauer. Proteins Struct. Funct. Genet. 1:43–46 (1986).
P. J. Carter, G. Winter, A. J. Wilkinson, and A. R. Fersht. Cell 38:835–840 (1984).
B. W. Matthews, H. Nicholson, and W. J. Becktel. Proc. Natl. Acad. Sci. USA 84:6663–6667 (1987).
G. N. Ramachandran and V. Sasisekharan. Adv. Protein Chem. 23:283–437 (1968).
S. K. Burley and G. A. Petsko. Science 225:23–28 (1985).
S. K. Burley and G. A. Petsko. Adv. Protein Chem. 39:125–189 (1988).
J. T. Kellis, Jr., K. Nyberg, D. Sali, and A. R. Fersht. Nature 333:784–786 (1988).
M. Matsumura, W. J. Becktel, and B. W. Matthews. Nature 334:406–410 (1988).
T. Alber, S. Dao-pin, K. Wilson, J. A. Wozniak, S. P. Cook, and B. W. Matthews. Nature 330:41–46 (1987).
J. F. Reidhaar-Olson and R. T. Sauer. Science 241:53–57 (1988).
M. L. Elwell and J. A. Schellman. Biochim. Biophys. Acta 494:367–383 (1977).
M. H. Hecht, J. M. Sturtevant, and R. T. Sauer. Proc. Natl. Acad. Sci. USA 81:5685–5689 (1984).
D. Shortle and A. K. Meeker. Proteins Struct. Funct. Genet. 1:81–89 (1986).
D. Shortle and A. K. Meeker. Biochemistry 28:936–944 (1989).
H. Nicholson, W. J. Becktel, and B. W. Matthews. Nature 336:651–656 (1988).
R. Hawkes, M. G. Grutter, and J. Schellman. J. Mol. Biol. 175:195–212 (1984).
M. Grutter, R. Hawkes, and B. Matthews. Nature 277:667–669 (1979).
W. J. Becktel, W. A. Baase, B. L. Chen, D. C. Muchmore, C. G. Schellman, and J. A. Schellman. Biophys. J. 49:572a (1986).
M. Grutter and B. Matthews. J. Mol. Biol. 154:525–535 (1982).
T. Alber, S. Dao-pin, J. A. Nye, D. C. Muchmore, and B. W. Matthews. Biochemistry 26:3754–3758 (1987).
B. W. Matthews. Biochemistry 26:6885–6887 (1987).
W. G. J. Hol, P. T. van Duijnen, and H. J. C. Berendsen. Nature 273:443–446 (1978).
W. G. J. Hol. Prog. Biophys. Mol. Biol. 45:149–195 (1985).
W. G. J. Hol. Angew. Chem. Int. Ed. Engl. 25:767–778 (1986).
W. G. J. Hol, L. M. Halie, and C. Sander. Nature 294:532–536 (1981).
R. P. Sheridan, R. M. Levy, and F. R. Salemme. Proc. Natl. Acad. Sci. USA 80:4545–4549 (1982).
K.-C. Chou, G. M. Maggiora, G. Nemethy, and H. A. Scheraga. Proc. Natl. Acad. Sci. USA 85:4295–4299 (1988).
J. S. Richardson. Adv. Protein Chem. 34:167–339 (1981).
P. S. Kim and R. L. Baldwin. Nature 307:329–334 (1984).
K. R. Shoemaker, P. S. Kim, D. N. Brems, S. Marqusee, E. J. York, I. M. Chaiken, J. M. Stewart, and R. L. Baldwin. Proc. Natl. Acad. Sci. USA 82:2349–2353 (1985).
C. Mitchinson and R. L. Baldwin. Proteins Struct. Funct. Genet. 1:23–33 (1986).
K. G. Strehlow and R. L. Baldwin. Biochemistry 28:2130–2133 (1989).
S. Marqusee and R. L. Baldwin. Proc. Natl. Acad. Sci. USA 84:8898–8902 (1987).
H. A. Scheraga. Proc. Natl. Acad. Sci. USA 82:5585–5587 (1985).
M. F. Perutz and G. Fermi. Proteins Struct. Funct. Genet. 4:294–295 (1988).
P. C. Lyu, L. A. Marky, and N. R. Kallenbach. J. Am. Chem. Soc. 111:2733–2734 (1989).
R. J. Abraham, B. D. Hudson, W. A. Thomas, and A. Krohn. J. Mol. Graph. 4:28–32 (1986).
D. Sali, M. Bycroft, and A. R. Fersht. Nature 335:740–743 (1988).
W. J. Becktel, W. A. Baase, R. Wetzel, and L. J. Perry. Biophys. J. 49:109a (1986).
M. H. Hecht, K. M. Hehir, H. C. M. Nelson, J. M. Sturtevant, and R. T. Sauer. J. Cell. Biochem. 29:217–224 (1985).
J. E. Villafranca, E. E. Howell, D. H. Voet, M. S. Strobel, R. C. Ogden, J. N. Abelson, and J. Kraut. Science 222:782–788 (1983).
J. A. Wells and D. B. Powers. J. Biol. Chem. 261:6564–6570 (1986).
M. W. Pantoliano, R. C. Ladner, P. N. Bryan, M. L. Rollence, J. F. Wood, and T. L. Poulos. Biochemistry 26:2077–2082 (1987).
R. T. Sauer, K. Hehir, R. S. Stearman, M. A. Weiss, A. Jeitler-Nilsson, E. G. Suchanek, and C. O. Pabo. Biochemistry 25:5992–5998 (1986).
M. Matsumura and B. W. Matthews. Science 243:792–794 (1989).
L. J. Perry and R. Wetzel. Science 226:555–557 (1984).
L. J. Perry and R. Wetzel. Biochemistry 25:733–739 (1986).
M. G. Mulkerrin, L. J. Perry, and R. Wetzel. In D. L. Oxender (ed.), Protein Structure, Folding, and Design, Alan Liss, New York, 1986, pp. 297–305.
G. McLendon and E. Radany. J. Biol. Chem. 253:6335–6337 (1978).
T. J. Ahern, J. I. Casal, G. A. Petsko, and A. M. Klibanov. Proc. Natl. Acad. Sci. USA 84:675–679 (1987).
P. T. Wingfield, R. J. Mattaliano, H. R. MacDonald, S. Craig, G. M. Clore, A. M. Gronenborn, and U. Schmeissner. Protein Eng. 1:413–417 (1987).
A. Abuchowski. J. Cell. Biochem. 11A:174 (1987).
P. Koziej, M. Mutter, H.-U. Gremlich, and G. Hölzemann. Z. Naturforsch. 40B:1570–1574 (1985).
M. Mutter, H. Mutter, R. Uhlmann, and E. Bayer. Biopolymers 15:917–927 (1976).
P. V. N. Rajasekharan and M. Mutter. Acc. Chem. Res. 14:122–130 (1981).
A. A. Ribiero, R. P. Saltman, M. Goodman, and M. Mutter. Biopolymers 21:2225–2239 (1982).
M. Hashimoto, K. Takada, Y. Kiso, and S. Muanishi. Pharm. Res. 6:171–176 (1989).
D. D. Chow and K. J. Hwang. J. Pharm. Sci. 76:S49 (1987).
E. R. Jakoi, P. E. Ross, H. P. Ting-Beall, B. Kaufman, and T. C. Vanaman. J. Biol. Chem. 262:1300–1304 (1987).
D. A. Towler, S. R. Eubanks, D. S. Towery, S. P. Adams, and L. Glaser. J. Biol. Chem. 262:1030–1036 (1987).
Y. A. Ovchinnikov, N. G. Abdulaev, and A. S. Bogachuk. FEBS Lett. 230:1–5 (1988).
F. S. Qaw and J. M. Brewer. Mol. Cell. Biochem. 71:121–127 (1986).
A. T. Fojo, P. L. Whitney, and M. W. Awad, Jr. Arch. Biochem. Biophys. 224:636–642 (1983).
P. Cujo, W. El-Deiry, P. L. Whitney, and W. M. Awad, Jr. J. Biol. Chem. 255:10828–10833 (1980).
R. Wolfenden, L. Anderson, P. M. Cullis, and C. C. B. Southgate. Biochemistry 20:849–855 (1981).
R. Desrosiers and R. M. Tanguay. J. Biol. Chem. 263:4686–4692 (1988).
R. M. Epand and K. E. Raymer. Int. J. Peptide Protein Res. 30:515–521 (1987).
H. Koide, S. Yokoyama, G. Kawai, J.-M. Ha, T. Oka, S. Kawai, T. Miyake, T. Fuwa, and T. Miyazawa. Proc. Natl. Acad. Sci. USA 85:6237–6241 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manning, M.C., Patel, K. & Borchardt, R.T. Stability of Protein Pharmaceuticals. Pharm Res 6, 903–918 (1989). https://doi.org/10.1023/A:1015929109894
Issue Date:
DOI: https://doi.org/10.1023/A:1015929109894