Abstract
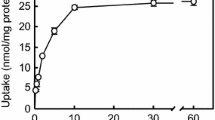
Two strains of Madin Darby canine kidney (MDCK) cells were grown on a polycarbonate membrane with 3-µm pores without any extracellular matrix treatment. The membrane, 2.45 cm in diameter, which is part of a commercially obtained presterilized culture insert, provides two chambers when placed in a regular six-well culture plate. This device was found to be convenient for investigating transport of a few selected fluid-phase markers across the MDCK cell monolayer. Both the strain from the American Type Culture Collection (ATCC) and the so-called highly resistant strain I, at a serial passage between 65 and 70, showed a seeding concentration-dependent lag phase followed by a growth phase with a 21-hr doubling time. When seeded at 5 × 104 cells/cm2, cell confluence was achieved in 5 days in a modified Eagle's minimum essential medium (MEM) containing 10% fetal bovine serum under a 5% CO2 atmosphere. Similarly, transepithelial electrical resistance (TEER) also reached a plateau value in 5 days. Both light and electron microscopic examinations revealed well-defined junctional structures. Transport of the fluid-phase markers, sucrose, lucifer yellow CH (LY), inulin, and dextran across the MDCK cell monolayers was studied primarily at 37°C following the apical-to-basolateral as well as the basolateral-to-apical direction. Large variations in the steady-state transport rate were observed for a given marker between the cell layer preparations. Thus, the present study proposes an “internal standard” procedure for meaningful comparisons of the transport rate. When normalized to the rate of sucrose, the rate ratio was 1.00:0.80:0.67:0.15 for sucrose:LY:inulin:dextran. This ratio was virtually independent of temperature, cell strain, direction of the marker migration, and TEER value, suggesting a common transport mechanism. The observed rate ratio appears to reflect molecular size and charge. The transport observed in the present study would consist, in theory, of both paracellular shunt and transcellular vesicular transport. Quantitative assessment of each transport mechanism in the overall transport has been difficult. The initial uptake of [3H]dextran estimated for the slowest transport observed in the present study was still 300-fold faster than a literature value. This appears to indicate that the transport observed in the present study is largely through the paracellular shunt pathway.
Similar content being viewed by others
REFERENCES
M. H. Friedman. Principles and Models of Biological Transport, Springer-Verlag, Berlin, 1986, pp. 134–157, 193–234.
J. M. Besterman and R. B. Low. Biochem. J. 210:1–13 (1983) (and references therein).
D. S. Misfeldt, S. T. Hamamoto, and D. R. Pitelka. Proc. Natl. Acad. Sci. USA 73:1212–1216 (1976).
M. Cereijido, E. S. Robbins, W. J. Dolan, C. A. Rotunno, and D. D. Sabatini. J. Cell Biol. 77:853–880 (1978).
J. C. W. Richardson, V. Scalera, and N. L. Simmons. Biochim. Biophys. Acta 673:26–36 (1981).
N. L. Simmons. J. Membrane Biol. 59:105–114 (1981).
D. Louvard. Proc. Natl. Acad. Sci. USA 77:4132–4136 (1980).
S. Fernandez-Castello, J. J. Bolivar, R. Lopez-Vancell, G. Beaty, and M. Cereijido. In M. Taub (ed.), Tissue Culture of Epithelial Cells, Plenum Press, New York, 1985, pp. 37–50.
C. H. von Bonsdorff, S. D. Fuller, and K. Simons EMBO J. 4:2781–2792 (1985).
A. M. Pitt, J. E. Gabriels, F. Badmington, J. McDowell, L. Gonzales, and M. E. Waugh. Biotech. 5:162–171 (1987).
R. F. Husted, M. J. Welch, and J. B. Stokes. Am. J. Physiol. 250:C214–C221 (1986).
J. A. Swanson, B. D. Yirinec, and S. C. Silverstein. J. Cell Biol. 100:851–859 (1985).
M. B. Furie, B. L. Naprstek, and S. C. Silverstein. J. Cell Sci. 88:161–175 (1987).
M. Taub and M. H. Saier, Jr. Methods Enzymol. LVIII:552–560 (1979).
L. Gonzalez-Mariscal, L. Borboa, R. Lopez-Vancell, G. Beaty, and M. Cereijido. In M. Taub (ed.), Tissue Culture of Epithelial Cells, Plenum Press, New York, 1985, pp. 25–36.
T. E. Phillips, T. L. Phillips, and M. R. Neutra. Cell Tissue Res. 247:547–554 (1987).
M. J. Buckmaster, D. L. Braico, A. L. Ferris, and B. Storrie. Cell Biol. Int. Rep. 11:501–507 (1987).
S. I. Rapoport. Blood-Brain Barrier in Physiology and Medicine, Raven Press, New York, 1976, p. 13.
J. L. Madara, D. Barenberg, and S. Carlson. J. Cell Biol. 102:2125–2136 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cho, M.J., Thompson, D.P., Cramer, C.T. et al. The Madin Darby Canine Kidney (MDCK) Epithelial Cell Monolayer as a Model Cellular Transport Barrier. Pharm Res 6, 71–77 (1989). https://doi.org/10.1023/A:1015807904558
Issue Date:
DOI: https://doi.org/10.1023/A:1015807904558