Abstract
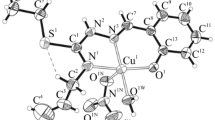
A bidentate and a quadridentate Schiff base having NS and NNSS donor sequences were prepared by condensing S-benzyldithiocarbazate (NH2NHCSSCH2Ph) with 2,3-butanedione (1:1 and 1:2 mole ratio). NiII and CuII complexes of these ligands were studied and characterised by elemental analyses and various physico-chemical techniques. The nickel complexes, [Ni(NS)2] and [Ni(SNNS)], were diamagnetic with square-planar and five-coordinate structures, respectively. The copper complex was, however, pentacoordinated. The ligands and the complexes were screened for anticancer activity against T-lymphoblastic leukemic cells (CEM-SS) and colon cancer cells (HT-29). The NS Schiff base was strongly active against leukemic cells with a CD50 value of 2.05 μg cm−3. The nickel and copper complexes were found to be stronger antioxidants than Vitamin E.
Similar content being viewed by others
References
M.A. Ali and S.E. Livingstone, Coord. Chem. Rev., 13, 101 (1974).
M.A. Ali and M.T.H. Tarafder, J. Inorg. Nucl. Chem., 39, 1785 (1977).
M.A. Ali, C.M. Haroon, M. Nazimuddin, S.M.M. Majumder, M.T.H. Tarafder and M.A. Khair, Transition Met. Chem., 17, 133 (1992).
M.A. Ali, M.H. Kadir, M. Nazimuddin, S.M.H. Majumder, M.T.H. Tarafder and M.A. Khair, Ind. J. Chem., 27A, 1064 (1988).
M.A. Ali, S.M.G. Hossain, S.M.M.H. Majumder, M.N. Uddin and M.T.H. Tarafder, Polyhedron, 6, 1653 (1987).
M.A. Ali, M. Uddin, M.N. Uddin, D.A. Chowdhury and M.T.H. Tarafder, Ind. J. Chem., 25A, 238 (1986).
M.A. Ali, S.M.M. Majumder, R.J. Butcher, J.P. Jasinski and J.M. Jasinski, Polyhedron, 16, 2749 (1997).
M.T.H. Tarafder and M. Rahim, Ind. J. Chem., 28A, 1105 (1989).
Y.P. Tian, C.Y. Duan and X.Z. You, Transition Met. Chem., 23, 17 (1998).
Y.P. Tian, C.Y. Duan, Z.L. Lu and X.Z. You, Polyhedron, 15, 2263 (1996).
X.H. Zhu, S.H. Liu, Y.J. Liu, J. Ma, C.Y. Duan, X.Z. You, Y.P. Tian, F.X. Xie and S.S. Ni, Polyhedron, 19, 181 (1998).
M.T.H. Tarafder, M.A. Ali, D.J. Wee, K. Azahari, S. Silong and K.A. Crouse, Transition Met. Chem., 25, 456 (2000).
M.T.H. Tarafder, M.A. Ali, N. Saravanan, W.Y. Weng, Saravana Kumar, N. Umar-Tsafe and K.A. Crouse, Transition Met. Chem., 25, 295 (2000).
M.A. Ali, N.E. Ibrahim, R.J. Butcher, J.P. Jasinski, J.M. Jasinski and J.C. Bryan, Polyhedron, 17, 1803 (1998).
M.A. Ali, K.K. Dey, M. Nazimuddin, F.E. Smith, R.J. Butcher, J.P. Jasinski and J.M. Jasinski, Polyhedron, 5, 3331 (1996).
M.A. Ali, M. Nazimuddin, R. Shaha, R.J. Butcher and J.C. Bryan, Polyhedron, 17, 3955 (1998).
M.A. Ali and S.G. Teoh, J. Inorg. Nucl. Chem., 40, 2013 (1978).
S. Bhattacharjee and R. Bhattacharyya, J. Chem. Soc., Dalton Trans., 1357 (1992).
A.G. Bingham, H. Bogge, A. Muller, E.W. Ainscough and A.M. Brodie, J. Chem. Soc., Dalton Trans., 493 (1987).
C.Y. Duan, Y.P. Tian, X.Z. You and T.C.W. Mak, Polyhedron, 16, 4097 (1997).
S.M.M.H. Majumder, M.A. Ali, F.E. Smith and M.A.U. Mridha, Polyhedron, 7, 2183 (1998).
M.E. Hossain, M.N. Alam, M.A. Ali, M. Nazimuddin, F.E. Smith and R.C. Hynes, Polyhedron, 15, 973 (1996).
S. Gou, X. You, Z. Xu, K. Yu and Z. Zhou, Polyhedron, 10, 2659 (1991).
M.E. Hossain, M.N. Alam, J. Begum, M.A. Ali, M. Nazimuddin, F.E. Smith and R.C. Hynes, Inorg. Chim. Acta, 249, 207 (1996).
F. Shahidi, Natural Antioxidants, AOCS Press, University of Newfoundland, Canada, 1997, p. 1.
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
M.A. Ali, I. Intan-Saflnar, M.M. Mackeen, S.H. El-Sharkawy, K. Takahata, H. Kanzaki and K. Kawazu, Natural Product Sciences, 4, 180 (1998).
A.W. Bauer, M.D.K. Kirby, J.C. Sherris and M. Turck, Am. J. Clin. Path., 45, 493 (1966).
C.D. Hufford and A.M. Clark, in Atta-ur-Rahman, (Ed), Studies in Natural Products Chemistry, Elsevier, Amsterdam, 1988, p. 421.
H. Kikuzaki and N. Nakatani, J. Food Science, 58, 1407 (1993).
A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier Publishing Co., Amsterdam, 1984.
W.T. Shier, Mammalian Cell Culture on $5 a Day: A Laboratory Manual of Low Cost Methods, Los Banos, University of the Philippines, 1991, p. 64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tarafder, M.T.H., Saravanan, N., Crouse, K.A. et al. Coordination chemistry and biological activity of nickel(II) and copper(II) ion complexes with nitrogen–sulphur donor ligands derived from S-benzyldithiocarbazate (SBDTC). Transition Metal Chemistry 26, 613–618 (2001). https://doi.org/10.1023/A:1012047001167
Issue Date:
DOI: https://doi.org/10.1023/A:1012047001167