Abstract
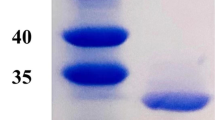
L-asparaginase EC 3.5.1.1 was purified to homogeneity from Thermus thermophilus. The apparent molecular mass of L-asparaginase by SDS-PAGE was found to be 33 kDa, whereas by its mobility on Sephacryl S-300 superfine column was around 200 kDa, indicating that the enzyme at the native stage acts as hexamer. The purified enzyme showed a single band on acrylamide gel electrophoresis with pI = 6.0. The optimum pH was 9.2 and the Km for L-asparagine was 2.8 mM. It is a thermostable enzyme and it follows linear kinetics even at 77°C. Chemical modification experiments implied the existence of histidyl, arginyl and a carboxylic residues located at or near active site while serine and mainly cysteine seems to be necessary for active form.
Similar content being viewed by others
References
Broome JD: Evidence that the L-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature 119: 1114–1115, 1961
Adamson RH, Fabro S: Antitumor activity and other biologic properties of L-asparaginase. Cancer Chemother Rept 52: 617–626, 1968
Haley EE, Fischer GA, Welch AD: The requirement for L-asparagine of mouse leukemia cells L5178Y in culture. Cancer Res 21: 532–536, 1961
Capizzi RL, Bertino GR, Handschumacher RE: L-asparaginase. Ann Rev Med 21: 433–434, 1970
Wriston JC Jr, Yellin TD: L-asparaginase: A review. Adv Enzymol 39: 185–248, 1973
Holcenberg JS: Enzyme therapy of cancer, future studies. Cancer Treat Rep 65: 61–65, 1981
Kafkewitz D, Bendich A: The inhibition of lymphocyte mitogenesis by asparaginase: A still unexplained phenomenon. Experientia 40: 1173–1177, 1984
Triantafillou DJ, Georgatsos JG, Kyriakidis DA: Purification and properties of a membrane-bound L-asparaginase of Tetrahymena pyriformis. Mol Cell Biochem 81: 43–51, 1988
Tsirka SAE, Kyriakidis DA: In vitro alterations of L-asparaginase activity of Tetrahymena pyriformis by lipids. Mol Cell Biochem 83: 147–155, 1988
Claasen E, Van Rooijen NA: Comparative study on the effectiveness of various procedures for attachment of two proteins (L-asparaginase and horse radish peroxidase) to the surface of liposomes. Prep Biochem 13: 167–174, 1983
Fishmann Y, Citri N: L-Asparaginase entrapped in liposomes: Preparation and properties. FEBS Lett 60: 17–20, 1975
Tsirka SAE, Kyriakidis DA: A model for the regulation of the activity of L-asparaginase/kinase enzyme of Tetrahymena pyriformis. Biochem Intern 19: 9–17, 1989
Tsirka SAE, Kyriakidis DA: L-Asparaginase of Tetrahymena pyriformis is associated with a kinase activity. Mol Cell Biochem 95: 77–87, 1990
Wriston JC Jr: Asparaginase. In: L. Lorand, S.P. Colwick, N.O. Kaplan (eds). Methods in Enzymology. Academic Press, New York, 1985, pp 608–618
Ho PKP, Milikin BE, Bobbit LJ, Grinnan LE, Burck JP, Frank HB, Boech DLV, Squires RW: Crystalline L-asparaginase from Escherichia coli. Purification and chemical characterization. J Biol Chem 245: 3708–3715, 1970
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Bearden JC: Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta 533: 525–529, 1978
Goldstein IJ, Hollerman CE, Merrick JM: Protein-carbohydrate interaction. I. The interaction of polysaccharides with concanavalin A. Biochim Biophys Acta 97: 68–76, 1967
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680–685, 1970
Blum H, Beier H, Gross HJ: Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels electrophoresis 8: 93–99, 1987
Laas T, Fast-Johanson A: Isoelectric focusing with Pharmalyte TM in gel rods. In: H. Peeters (ed). Protides of the Biological Fluids. Pergamon Press, Oxford, 1979, pp 693–697
Kameshita I, Fujisawa H: A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfatepolyacrylamide gel. Anal Biochem 183: 139–143, 1989
Davidson L, Brear DR, Wingard P, Hawkins J, Kitto GB: Purification and properties of L-glutaminase-L-asparaginase from Pseudomonas acidovorans. J Bacteriol 129:1379–1386, 1977
Distasio JA, Niederman RA, Kafkewitz D, Goodman D: Purification and characterization of L-asparaginase with anti-lymphoma activity from Vibrio succinogenes. J Biol Chem 251: 6929–6933, 1976
Peterson RE, Ciegler A: L-asparaginase production by Erwinia aroidea. Appl Microbiol 18: 64–67, 1969
Jerebzoff-Quintin S, Jerebzoff S: L-asparaginase activity in Leptosphaeria michotii. Isolation and properties of two forms of the enzyme. Physiol Plant 64: 74–80, 1985
Tsavdaridis IK, Triantafillidou DC, Kyriakidis DA: Two forms of Lasparaginase in Tetrahymena thermophila. Biochem Mol Biol Int 32: 67–77, 1994
Jerebzoff-Quintin S, Jerebzoff S: Reversible phosphorylation of asparaginase complex in Leptosphaeria michotii: Characterization of associated protein kinase and protein phosphatase activities. Biochem Biophys Res Commun 140: 1135–1142, 1986
Miles EW: Modification of histidyl residues in proteins by diethylpyrocarbonate. Meth Enzymol 47: 431–442, 1977
Melcior WB, Fahrey D: Ethoxyformylation of proteins. Reaction of ethoxyformic anhydrite with alpha-chymotrypsin, pepsin and pancreatic ribonuclease. Biochemistry 9: 251–258, 1970
Means GE, Feeney RE: Acylating and similar reagents. In: Chemical Modification of Proteins. Holden-Day Inc., San Francisco, California, 1971
De la Mata I, Castillon MP, Dominguez JM, Macarron R, Acebal C: Chemical modification of beta-glycosidase from Trichoderma reesei QM 9414. J Biochem. 114: 754–759, 1993
Belleau B, Malek G: A new convenient reagent for peptide syntheses. J Am Chem Soc. 90: 1651–1652, 1968
Zhang S, McCarter JD, Okamuta-Oho Y, Yaghi F, Hinek A, Withers SG, Callahan JW: Kinetic mechanisms and characterization of human beta-galactosidase precursor secreted by permanently transfected Chinese hamster ovary cells. Biochem J 304: 281–288, 1994
Manna S, Sinha A, Sadhukhan R, Chakrabarty SL: Purification, characterization and antitumor activity of L-asparaginase isolated from Pseudomonas strutzeri MB-405. Curr Microbiol 30: 291–298, 1995
Kyriakidis DA, Tsirka SAE, Tsavdaridis IK, Iliadis SN, Kortsaris AH: Antiproliferative activity of L-asparaginase of Tetrahymena pyriformis on human breast cancer cell lines. Mol Cell Biochem 96: 137–142, 1990
Schwartz RS: Immunosuppression by L-asparaginase. Nature 224: 275–276, 1969
Distasio JA, Salazar AM, Nadji M, Durden DL: Glutaminase-free asparaginase from Vibrio succinogenes. An antilymphoma enzyme lacking hepatotoxicity. Int J Cancer 30: 343–347, 1982
Raha SK, Roy SK, Dey SK, Chakrabarty SL: Purification and properties of an L-asparaginase from Cylidrocarbon obtusisporum MB-10. Biochem Int 21: 987–1000, 1990
Bagert U, Rohm K-H: On the role of histidine and tyrosine residues in E. coli asparaginase. Chemical modification and 1H-nuclear magnetic resonance studies. Biochim Biophys Acta 999: 36–41, 1989
Derst C, Wehner A, Specht V, Rohm KH: States and functions of tyrosine residues in Escherichia coli asparaginase II. Eur J Biochem 224: 533–540, 1994
Wehner A, Harms E, Jennings MP, Beacham IR, Derst C, Bast P, Rohm KH: Site-specific mutagenesis of Escherichia coli asparaginase II. None of the three histidine residues is required for catalysis. Eur J Biochem 208: 475–480, 1992
Harms E, Wehner A, Aung H-P, Rohm KH: A catalytic role for threonine-12 of in E. coli asparaginase II as established by site-directed mutagenesis. FEBS Lett 285: 55–58, 1991
Palm GJ, Lubkowski J, Derst C, Schleper S, Rohm KH, Wlodawer A: A covalently bound catalytic intermediate in Escherichia coli asparaginase: Crystal structure of a Thr-89–Val mutant. FEBS Lett 390: 211–216, 1996
Swain AL, Jaskolski M, Housset D, Mohana Rao JK: Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proc Natl Acad Sci 90: 1474–1478, 1993
Pritsa A, Choli-Papadopoulou T, Kyriakidis DA: Studies on the primary structure of L-asparaginase from Thermus thermophilus. J Prot Chem 17: 548–549, 1998
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pritsa, A.A., Kyriakidis, D.A. L-asparaginase of Thermus thermophilus: Purification, properties and identificaation of essential amino acids for its catalytic activity. Mol Cell Biochem 216, 93–101 (2001). https://doi.org/10.1023/A:1011066129771
Issue Date:
DOI: https://doi.org/10.1023/A:1011066129771