Abstract
Purpose. To study the pulmonary absorption and tolerability of various formulations of the decapeptide cetrorelix acetate in rats by a new aerosol delivery system (ASTA-ADS) for intratracheal application.
Methods. Using the ASTA-ADS, cetrorelix liquid formulations (aqueous solutions for ultrasonic nebulization) were firstly selected and subsequently delivered as nebulized aerosol to orotracheally cannulated rats. The pharmacologic effect (decrease of testosterone serum level) of four cetrorelix formulations was determined in rats by enzyme linked immunosorbant assay, and pharmacokinetic data were determined after measurement of cetrorelix serum level by radioimmunoassay. Histological examination of the lung was performed at the end of the experiments, and in a supplementary experiment the respiratory parameters (resistance and compliance) of rats were monitored by a validated pulmonary monitoring system during the aerosol application of the same formulations.
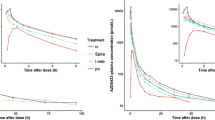
Results. After an exposure time of 5 min, the applied formulations reduced the testosterone concentration in serum to subnormal levels (≤1 ng/ml) over a period of 24 h. Comparing the plasma concentration after intratracheal aerosolization with data of intravenous administration, the mean calculated bioavailabilities for the four formulations using the corrected dose (delivered—exhaled amount) were between 48.4 ± 27.0% and 77.4 ± 44.0%. The histologic examination of the lungs revealed different tolerability of the various tested formulations ranging from locally intolerable to well tolerated. The measurement of the lung function parameters did not reveal any compound or formulation related changes.
Conclusions. Our studies show that cetrorelix can be effectively administered as aerosol and that intratracheal aerosolization via the ASTA-ADS provides results that are well comparable to other application routes, as demonstrated by statistical comparison of the newly obtained data with previous results from intratracheal instillation of cetrorelix solutions in rats.
Similar content being viewed by others
REFERENCES
P. R. Byron. Determinants of drug and polypeptide bioavailability from aerosols delivered to the lung. Adv. Drug. Del. Rev. 5:107-132 (1990).
A. L. Adjei and P. K. Gupta. Pulmonary delivery of therapeutic peptides and proteins. J. Control. Release 29:361-373 (1994).
J. Yu and Y. W. Chien. Pulmonary drug delivery: Physiologic and mechanistic aspects. Ther. Drug Car. Syst. 14:395-453 (1997).
J. S. Patton. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 19:3-36 (1996).
A. Adjei, R. Doyle, M. Pratt, R. Finley, and E. Johnson. Bioavailability of leuprolide following intratracheal administration to beagle dogs. Int. J. Pharm. 61:135-144 (1990).
P. L. Smith, J. Marcello, D. C. Chiossone, D. Orner, and I. J. Hildalgo. Absorption of an RGD peptide (SK & F 107760) following intratracheal administration in rats. Int. J. Pharm. 106:95-101 (1994).
F. Komada, S. Iwakawa, N. Yamamoto, H. Sakakibara, and K. Okumura. Intratracheal delivery of peptide and protein agents: Absorption from solution and dry powder by rat lung. J. Pharm. Sci. 83:863-867 (1994).
R. Lizio, T. Klenner, G. Borchard, P. Romeis, A. W. Sarlikiotis, and C.-M. Lehr. Systemic delivery of the GnRH antagonist cetrorelix by intratracheal instillation in anesthetized rats. Eur. J. Pharm. Sci. 9:253-258 (2000).
G. Oberdörster, C. Cox, and R. Gelein. Intratracheal instillation vs intratracheal inhalation of tracer particles for measuring lung clearance function. Exp. Lung Res. 23:17-34 (1997).
M. Osaier and G. Oberdörster. Intratracheal instillation vs intratracheal inhalation: Differences in particle effects. Fund. Appl. Tox. 40:220-227 (1997).
J. D. Brain and P. A. Valberg. State of the art. Deposition of aerosol in the respiratory tract. Am. Rev. Resp. Dis. 120:1325-1373 (1979).
T. D. Sweeney and J. D. Brain. Pulmonary deposition: Determinants and measurement techniques. Tox. Pathol. 19:384-397 (1991).
R. Lizio, D. Marx, T. Nolte, C.-M. Lehr, A. W. Sarlikiotis, G. Borchard, W. Jahn, and T. Klenner. Development of a new aerosol delivery system for systemic pulmonary delivery in anesthetized and orotracheal cannulated rats. Laboratory Animals (in press).
S. Bajusz, M. Kovacs, M. Gazdag, L. Bokser, T. Karashima, V. J. Csernus, T. Janaky, J. Guoth, and A. V. Schally. Highly potent antagonists of luteinizing hormone-releasing hormone (LHRH) free of edematogenic effects. Proc. Natl. Acad. Sci. USA 85:1637-1641 (1988).
A. V. Schally. The use of LHRH analogs in gynecology and tumor therapy. In P. Belfort, J. A. Pinotti, and T. K. A. B. Eskes (eds.), Advances in Gynecology and Obstetrics Vol. 6 General Gynecology, Parthenon Publ., Carnfort, 1989 pp. 3-20.
H. Schreier, J. Engel, J. J. McNicol, H. Derendorf, K. Groot, and A. V. Schally. Systemic delivery of the luteinizing hormone-releasing hormone antagonist cetrorelix (SB-75) via pulmonary instillation in the unanesthetized awake sheep. Eur. J. Pharm. Sci. 2:303-306 (1994).
K. M. G. Taylor and O. N. M. McCallion. Ultrasonic nebulizer for pulmonary drug delivery. Int. J. Pharm. 153:93-104 (1997).
R. W. Niven, A. Y. Ip, S. Mittelman, S. J. Prestrelski, and T. Arakawa. Some factors associated with the ultrasonic nebulization of proteins. Pharm. Res. 12:53-59 (1995).
R. W. Niven, K. L. Whitcomb, L. Shaner, A. Y. Ip, and O. B. Kinstler. The pulmonary absorption of aerosolized and intratracheally instilled rhG-CSF and monoPEGylated rhG-CSF. Pharm. Res. 12:1343-1349 (1995).
R. M. Evans, S. J. Farr, N. A. Armstrong, and S. M. Chatham. Formulation and in vitro evaluation of pressurized inhalation aerosols containing isotropic system of lecithin and water. Pharm. Res. 8:629-635 (1990).
R. Lizio, A. Westhof, C.-M. Lehr, and T. Klenner. Oral endotracheal intubation of rats for instillation and aerosol delivery. Laboratory Animals (in press).
I. Kohler, R. Meier, A. Busato, G. Neiger-Aeschbacher, and U. Schatzmann. Is carbon dioxide (CO2) a useful short acting anaesthetic for small laboratory animals? Laboratory Animals 33:155-161 (1999).
W. Zeller, H. Weber, B. Panoussis, T. Bürge, and R. Bergmann. Refinement of blood sampling from the sublingual vein of rats. Laboratory Animals 32:369-376 (1998).
J. J. O'Neil and J. A. Raub. Pulmonary function testing in small laboratory mammals. Envir. Health Persp. 56:11-22 (1984).
C. J. Harvey, M. J. O'Doherty, C. J. Page, S. H. L. Thomas, T. O. Nunan, and D. F. Treacher. Comparison of jet and ultrasonic nebulizer pulmonary aerosol deposition during mechanical ventilation. Eur. Respir. J. 10:905-909 (1997).
K. E. Discroll, J. M. Carter, D. G. Hassenbein, and B. Howard. Cytokines and particle-induced inflammatory cell recruitment. Envir. Health Persp. 105:1159-1164 (1997).
D. Raeburn, S. L. Underwood, and M. E. Villamil. Techniques for drug delivery to the airway, and the assessment of lung function in animal models. J. Pharmacol. Toxicol. Meth. 27:143-159 (1992).
Y. Li, Z. Shao, D. B. DeNicola, and A. K. Mitra. Effect of a conjugated bile salt on the pulmonary absorption of insulin in rats. Eur. J. Biopharm. 39:216-221 (1993).
J. Shen, K. J. Elbert, F. Yamashita, C.-M. Lehr, K.-J. Kim, and V. H. L. Lee. Organic cation transport in rabbit alveolar epithelial cell monolayers. Pharm. Res. 16:1280-1287 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lizio, R., Klenner, T., Sarlikiotis, A.W. et al. Systemic Delivery of Cetrorelix to Rats by a New Aerosol Delivery System. Pharm Res 18, 771–779 (2001). https://doi.org/10.1023/A:1011028227155
Issue Date:
DOI: https://doi.org/10.1023/A:1011028227155