Abstract
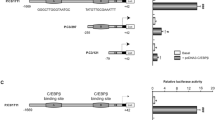
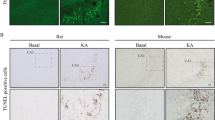
Signal transduction pathways that mediate neuronal commitment to apoptosis involve the nuclear factor kappa B (NF-κB) transcription factor. The bcl-x gene is a member of the bcl-2 family of genes that regulate apoptosis, and gives rise to two proteins, Bcl-XL and Bcl-XS, via alternative mRNA splicing. Bcl-XL protein, like Bcl-2, is a dominant inhibitor of apoptotic cell death, whereas Bcl-XS promotes apoptosis. While there is high expression of Bcl-XL in the developing and adult brain, few transcriptional control elements have been identified in the bcl-x promoter. There are two functional nuclear factor-kappa B (NF-κB) DNA binding sites clustered upstream of the brain-specific transcription start site in the upstream promoter region of murine bcl-x. Recombinant NF-κB proteins bind to these sites. Also NF-κB overexpression, coupled with bcl-x promoter/reporter assays using a series of murine bcl-x promoter and deletion mutants, has identified the downstream 1.1kb of the bcl-x promoter as necessary for basal promoter activity and induction by NF-κB in support of the hypothesis that NF-κB can act to enhance Bcl-XL expression via highly selective interactions with the bcl-x promoter, where NF-κB binding and promoter activation are dependent on specific DNA binding site sequences and NF-κB protein dimer composition. Hypoxia induces apoptosis in the hippocampus where the NF-κB dimers c-Rel/p50 and p50/p50 bind to the bcl-x promoter NF-κB site.
Similar content being viewed by others
REFERENCES
Cotman, C., Whittemore, E., Watt, J., Anderson, A. J., and Loo, D. 1994. Possible role of apoptosis in Alzheimer's disease. Annu. NY Acad. Sci. 747:36–49.
Thompson, C. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462.
Kerr, J., Wyllie, A., and Currie, A. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit. J. Cancer 26:239–257.
Sulston, J. and Horvitz, H. 1977. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Develop. Biol. 82:110–156.
Ellis, H. and Horvitz, H. 1986. Genetic control of programmed cell death in the nematode C. elegans. Cell 44:817–829.
Hengartner, M. and Horvitz, H. 1992. C. elegans gene ced-9 protects cells from programmed cell death. Nature 356:494–499.
Yuan, J., Shaham, S., Ledoux, S., Ellis, H., and Horvitz, H. 1993. The C. elegans death gene ced-3 encodes a protein similar to mammalian interleukin-1-beta-converting enzyme. Cell 75:641–652.
Lazebnik, Y., Kaufmann, S., Desnoyers, S., Poirier, G., and Earnshaw, W. 1994. Cleavage of poly(ADP-ribose)polymerase by a proteinase with properties like ICE. Nature 371:346–347.
Nicholson, D., Ali, A., Thornberry, N., Vaillancourt, J., Ding, C., Gallant, M., Gareau, Y., Griffin, P., Labelle, M., and Lazebnik, Y. 1995. ICE/CED-3 necessary for mammalian apoptosis. Nature 376:37–43.
Xue, D. and Horvitz, H. 1995. Inhibition of the Caenorhabditis elegans cell death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature 377:248–251.
Yuan, J. and Horvitz, H. 1992. The Caenorhabditis elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development 116:309–320.
Zou, H., Henzel, W., Liu, X., Lutschg, A., and Wang, X. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413.
Tsujimoto, Y. and Croce, C. 1986. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Nat. Acad. Sci. USA 83:5214–5218.
Hengartner, M. and Horvitz, H. 1994. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76:665–676.
Ashkenazi, A. and Dixit, V. 1998. Death Receptors: Signaling and Modulation. Science 281:1305–1308.
Thornberry, N., Rosen, A., and Nicholson, D. 1997. Control of apoptosis by proteases. Adv. Pharmcol. 41:155–177.
Nunez, G., Benedict, M., Hu, Y., and Inohara, N. 1998. Caspases: the proteases of the apoptotic pathway. Oncogene 17:3237–3245.
Alnemri, E., Livingston, D., Nicholson, D., Salveson, G., Thornberry, N., Wong, W., and Yuan, J. 1996. Human ICE/CED-3 protease nomenclature. Cell 87:171.
Walker, N., Talanian, R., Brady, K., Dang, L., Bump, N., Ferenz, C., Franklin, S., Ghayur, T., Hackett, M., Hammill, L., Herzog, L., Hugunin, M., Houy, W., Mankovich, J., Mc-Guiness, L., Orlewicz, E., Paskind, M., Pratt, C., Reis, P., Summani, A., Terranova, M., Welch, J., Xiong, L., Moller, A., Tracey, D., Kamen, R., and Wong, W. 1994. Crystal structure of the cysteine protease interleukin-1-beta-converting enzyme: a (p20/p10)2 homodimer. Cell 78:342–352.
Wilson, K., Black, J.-A., Thomson, J., Kim, E., Griffith, J., Navia, M., Murcko, M., Chambers, S., Aldape, R., Raybuck, S., and Livingston, D. 1994. Structure and mechanism of interleukin-1-beta-converting enzyme. Nature 370:270–275.
Rotonda, J., Nicholson, D., Fazil, K., Gallant, M., Gareau, Y., Labelle, M., Peterson, E., Rasper, D., Ruel, R., Vaillancourt, J., Thornberry, N., and Becker, J. 1996. The three dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nature Struct. Biology 3:619–625.
Cohen, G. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1–16.
Xue, D. and Horvitz, H. 1997. Caenorhabditis elegans CED-9 is a bifunctional cell death inhibitor. Nature 377:248–251.
Cheng, E., Kirsch, D., Clem, R., Ravi, R., Kastan, M., Bedi, A., Ueno, K., and Hardwick, J. 1997. Conversion of Bcl-2 to a Bax-like Death Effector by Caspases. Science 278:1966–1968.
Clem, R., Cheng, E., Karp, C., Kirsch, D., Ueno, K., Takahashi, A., Kastan, M., Griffin, D., Earnshaw, W., Veliuona, M., and Hardwick, J. 1998. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Nat. Acad. Sci. 95:554–559.
Widmann, C., Gibson, S., and Johnson, G. 1998. Caspasedependent cleavage of signalling proteins during apoptosis: A turn-off mechanism for anti-apoptotic signals. J. Biol. Chem. 273:7141–7147.
Wen, L.-P., Fahrni, J., Troie, S., Guan, J.-L., Orth, K., and Rosen, G. 1997. Cleavage of focal adhesion kinase by caspases during apoptosis. J. Biol. Chem. 272:26056–26061.
Rao, L., Perez, D., and White, E. 1996. Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol. 135:1441–1455.
Brancolini, C., Benedetti, M., and Schneider, C. 1995. Microfilament reorganization during apoptosis: The role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 14:5179–5190.
Kothakota, S., Azuma, T., Reinhard, C., Klippel, A., Tang, J., Chu, K., TJ, M., Kirschner, M., Koths, K., Kwiatkowski, D., and Williams, L. 1997. Caspase-3-generated fragment of gelsolin: Effector of morhological change in apoptosis. Science 278:294–298.
Cryns, V., Bergeron, L., Zhu, H., Li, H., and Yuan, J. 1996. Specific cleavage of alpha-fodrin during fas-and TNF-induced apoptosis is mediated by an interleukin-1-beta-converting enzyme/ced-3 protease distinct from the poly(ADP ribose)polymerase protease. J. Biol. Chem. 271:31277–31282.
Sakahira, H., Enari, M., and Nagata, S. 1998. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391:96–99.
Chinnaiyan, A., O'Rourke, K., Yu, G.-L., Lyons, R., Garg, M., Duan, D., Xing, L., Gentz, R., Ni, J., and Dixit, V. 1996. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274:990–992.
Chinnaiyan, A., O'Rourke, K., Tewari, M., and Dixit, V. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505–512.
Hsu, H., Xiong, J., and Goeddel, D. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495–504.
Muzio, M., Chinnaiyan, A., Kischkel, F., O'Rourke, K., Shevchenko, A., Ni, J., Scaffidi, C., Bretz, J., Zhang, M., Gentz, R., Mann, M., Kreammer, P., Peter, M., and Dixit, V. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death inducing signaling complex. Cell 85:817–827.
Martin, D., Siegel, R., Zheng, L., and Lenardo, M. 1998. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHa1) death signal. J. Biol. Chem. 273:4345–4349.
Muzio, M., Stockwell, B., Salvesen, G., and Dixit, V. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926–2930.
Fernandez-Alnemri, T., Armstrong, R., Krebs, J., Srinivasula, S., Wang, L., Bullrich, F., Fritz, L., Trapani, J., Tomaselli, K., Litwack, G., and Alnemri, E. 1996. In vitro activation of CPP32 and Mch3 by Mch4, a novel humna apoptotic cysteine protease containing two FADD-like domains. Proc. Nat. Acad. Sci. USA 93:7464–7469.
Liu, X., Kim, C., Yang, J., Jemmerson, R., and Wang, X. 1996. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86:147–157.
Li, P., Nijhawan, D., I, B., Srinivasula, S., Ahmad, M., Almeri, E., and Wang, X. 1997. Cytochrome C and dATP-dependent formation of Apaf-1/caspase-9 initiates an apoptotic protease cascade. Cell 91.
Zou, H., Li, Y., and Wang, X. 1999. An APAF-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274:11549–11556.
Zoratti, M. and Szabo, I. 1995. The mitochondrial permiability transition. Biochem. Biophys. Acta 1241:139–176.
Bernardi, P. and Petronilli, V. 1996. The permiability transition pore as a mitochondrial calcium release channel: a critical appraisal. J. Bioenerg. Biomem. 28:129–136.
Beutner, G., Riede, B., Welte, W., and Brdiczka, D. 1996. Complexes between kinases, mitochondrial porin, and adenylate translocator in rat brain resemble the permiability transition pore. FEBS Lett. 396:189–195.
Brustovetsky, N. and Klingenberg, M. 1996. Mitochondrial ADP/ATP can be reversably converted into a large channel by Ca2+. Biochemistry 35:8483–8488.
Vayssiere, J.-L., Petit, P., Risler, Y., and Mignotte, B. 1994. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Nat. Acad. Sci. USA 91:11752–11756.
Zamzami, N., Marchetti, P., Castedo, M., Zanin, C., Vayssiere, J.-L., Petit, P., and Kroemer, G. 1995. Reduction in mitochondrial potential constitutes an early irreversable step in programmed lymphocyte death in vivo. J. Exp. Med. 181:1161–1172.
Marchetti, P., Castedo, M., Susin, S., Zamzami, N., Hirsch, T., Macho, A., Haeffner, A., Hirsch, F., Geuskins, M., and Kroemer, G. 1996. Mitochondrial permiability transition is a central coordinating event of apoptosis. J. Exp. Med. 184:1155–1160.
Vander Heiden, M., Chandel, N., Schumacker, P., and Thompson, C. 1999. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3:159–167.
Korsmeyer, S. 1992. Bcl-2 initiates a new catagory of oncogenes: Regulators of cell death. Blood 80:879–886.
Bahkshi, A., Jenson, J., Goldman, P., Wright, J., McBride, O., Epstein, A., and Korsmeyer, S. 1985. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell 41:899–906.
Tsujimoto, Y., Gorham, J., Cossman, J., Jaffe, E., and Croce, C. 1985. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229:1390–1393.
Cleary, M., Smith, S., and Sklar, J. 1986. Cloning and structural analysis of cDNAs for bcl-2, and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47:19–28.
Graninger, W., Seto, M., Boutain, B., Goldman, P., and Korsmeyer, S. 1987. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J. Clin. Invest. 80:1512–1515.
Nunez, G., London, L., Hockenbery, D., Alexander, M., Mc-Kearn, J., and Korsmeyer, S. 1990. Deregulated Bcl-2 expression selectively prolongs survival of growth factor-deprived hematopoietic cell lines. J. Immunol. 144:3602–3610.
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R., and Korsmeyer, S. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334–336.
Boise, L., Gonzalez-Garcia, M., Postema, C., Ding, L., Lindsten, T., Turka, L., Mao, X., Nunez, G., and Thompson, C. 1993. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic c-ell death. Cell 74:597–608.
Boyd, J., Gallo, G., Elangovan, B., Houghton, A., Malstrom, S., Avery, B., Ebb, R., Subramanian, T., Chittenden, T., Lutz, R., and Chinnadurai, G. 1995. Bik, a novel death-inducing protein shares a distinct motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11:1921–1928.
Chittenden, T., Harrington, E., O'Connor, R., Flemminton, C., Lutz, R., Evan, G., and Guild, B. 1995. Induction of apoptosis by the Bcl-2 homolog Bak. Nature 374:733–736.
Yang, E., Zha, J., Jockel, J., Boise, L., Thompson, C., and Korsmeyer, S. 1995. Bad, a heterodimeric partner of Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285–291.
Gibson, L., Holmgreen, S., Huang, D., Bernard, O., Copeland, N., Jenkins, N., Sutherland, G., Baker, E., Adams, J., and Cory, S. 1996. Bcl-w, A novel member of the bcl-2 family, promotes cell survival. Oncogene 13:665–675.
Wang, K., Yin, X., Chao, D., Milliman, C., and Korsmeyer, S. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859–2869.
Sattler, M., Liang, H., Nettesheim, D., Meadows, R., Harlan, J., Eberstadt, M., Yoon, H., Shuker, S., Chang, B., Minn, A., Thompson, C., and Fesik, S. 1997. Structure of Bcl-x(L)-Bak peptide complex-recognition between regulators of apoptosis. Science 275:983–986.
Oltvai, Z., Milliman, C., and Korsmeyer, S. 1993. Bcl-2 Heterodimerizes In Vivo with a Conserved Homolog, Bax, That Accelerates Cell Death. Cell 74:609–619.
Zha, J., Harada, H., Osipov, K., Jockel, J., Waksman, G., and Korsmeyer, S. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3-not Bcl-xL. Cell 87:619–628.
Ito, T., Deng, X., Carr, B., and May, W. 1997. Bcl-2 Phosphorylation Required for Anti-apoptosis Function. J. Biol. Chem. 272:11671–11673.
Gross, A., Yin, X.-M., Wang, K., Wei, M., Jockel, J., Milliman, C., Erdjument-Bromage, H., Tempst, P., and Korsmeyer, S. 1999. Caspase Cleaved BID Targets Mitochondria and is required for Cytochrome c Release, while Bcl-xL Prevents This Release but Not Tumor Necrosis Factor-R1/Fas Death. J. Biol. Chem. 274:1156–1163.
Hsu, Y.-T., Wolter, K., and Youle, R. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-xL during apoptosis. Proc. Natl. Acad. Sci. USA 94:3668–3672.
Wolter, K., Hsu, Y., Smith, C., Nechushtan, A., Xi., X., and Youle, R. 1997. Movement of Bax from the cytosol to the mitochondria during apoptosis. J. Cell Biol. 139:1281–1292.
Gonzalez-Garcia, M., Perez-Ballestero, R., Ding, L., Duan, L., Boise, L., Thompson, C., and Nunez, G. 1994. Bcl-xL is the major bcl-x mRNA from expressed during murine development and its product localizes to mitochondria. Development 120:3033–3042.
Antonsson, B., Conti, F., Ciavatta, A., Montessuit, S., Lewis, S., Martinou, I., Bernasconi, L., Bernard, A., Mermod, J.-J., Mazzei, G., Maundrell, K., Gambale, F., Sadoul, R., and Martinou, J.-C. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science 277:370–372.
Minn, A., Velez, P., Schendel, S., Liang, H., Muchmore, S., Fesik, S., Fill, M., and Thompson, C. 1997. Bcl-x(l) forms an ion channel in synthetic lipid membranes. Nature 385:353–357.
Schendel, S., Xie, Z., Montal, M., Matsuyama, S., Montal, M., and Reed, J. 1997. Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA 94:5113–5118.
Schlesinger, P., Gross, A., Yin, X., Yamamoto, K., Saito, M., Waksman, G., and Korsmeyer, S. 1997. Comparison of the ion channel activity of pro-apoptotic BAX and anti-apoptotic BCL-2. Proc. Natl. Acad. Sci. USA 94:357–362.
Jurgensmeier, J., Xie, Z., Devereux, Q., Ellerby, L., Bredesen, D., and Reed, J. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997–5002.
Marzo, I., Brenner, C., Zamzami, N., Jurgensmeier, J., Susin, S., Vieira, H., Prevost, M.-C., Xie, Z., Matsuyama, S., Reed, J., and Kroemer, G. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondria control of apoptosis. Science 281:2027–2031.
Narita, M., Shimizu, S., Ito, T., Chittenden, T., Lutz, R., Matsuda, H., and Tsujimoto, Y. 1998. Bax interacts with the permiability transition pore to induce permiability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:14681–14686.
Shimizu, S., Eguchi, Y., Kamike, W., Funahashi, Y., Mignon, A., Lacronique, V., Matsuda, H., and Tsujimoto, Y. 1998. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. USA 95:1455–1459.
Shimizu, S., Narita, M., and Tsujimoto, Y. 1999. Bcl-2 family proteins regulate the release of cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487.
Finucane, D., Bossy-Wetzel, E., Waterhouse, N., Cotter, T., and Green, D. 1999. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 274:2225–2233.
Gonzalez-Garcia, M., Garcia, I., Ding, L., O'shea, S., and Boise, L. 1995. Bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent cell death. Proc. Natl. Acad. Sci. USA 92:4304–4308.
Motoyama, N., Wang, F., Roth, K., Sawa, H., and Nakayama, K.-I. 1995. Masive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506–1510.
Chao, D. and Korsmeyer, S. 1998. Bcl-2 Family: Regulators of cell death. Annu. Rev. Immunol. 16:395–419.
Hu, Y., Benedict, M., Wu, D., Inohara, N., and Nunez, G. 1998. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1 dependent caspase-9 activation. Proc. Natl. Acad. Sci. USA 95:4386–4391.
Pan, G., O'Rourke, K., and Dixit, V. 1998. Caspase-9, Bcl-XL, and Apaf-1 Form a Ternary Complex. J. Biol. Chem. 273:5841–5845.
Sen, R. and Baltimore, D. 1986. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705–716.
Beg, A., Sha, W., Bronson, R., Ghosh, S., and Baltimore, D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167–170.
Sonenshein, G. 1997. Rel/NF-kappa B transcription factors and the control of apoptosis. Seminars in Cancer Biol. 8:113–119.
Schmid, R., Perkins, N., Duckett, C., Andrews, P., and Nabel, G. 1991. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature 352:733–736.
Bours, V., Burd, P., Brown, K., J, V., Park, S., Ryseck, R.-P., Bravo, R., Kelly, K., and Siebenlist, U. 1992. A novel mitogeninducible gene product related to p50/p105-NF-kappa B participates in transactivation through a kappa B site. Mol. Cell. Biol. 12:685–695.
Mercurio, F., Didonato, J., Rosette, C., and Karin, M. 1992. Molecular cloning and characterization of a novel Rel/NFkappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA and Cell Biol. 11:523–537.
Bours, V., Villalobos, J., Burd, P., Kelly, K., and Siebenlist, U. 1990. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature 348:76–80.
Ghosh, S., Gifford, A., Riviere, L., Tempst, P., Nolan, G., and Baltimore, D. 1990. Cloning of the p50 DNA binding subunit of NF-kappa B; homolgy to rel and dorsal. Cell 62.
Kieran, M., Blank, V., Logeat, F., Vandekerckhove, J., Lottspeich, F., Le Bail, O., Urban, M., Kourilsky, P., Baeuerle, P., and Israel, A. 1990. The DNA-binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell 62:1007–1018.
Nolan, G., Ghosh, S., Liou, H.-C., Tempst, P., and Baltimore D. 1991. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell 64:961–969.
Ruben, S. M., Dillon, P. J., Schreck, R., Henkel, T., Chen, C.-H., Maher, M, Baeurle, P. A., and Rosen, C. A. 1991. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kB. Science 251:1490–1493.
Brownell, E., Mittereder, N., and Rice, N. 1989. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene 4:935–942.
Ryseck, R.-P., Bull, P., Takamiya, M., Bours, V., Siebenlist, U., Dobrzanski, P., and Bravo, R. 1992. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol. Cell. Biol. 12:674–684.
Liou, H.-C. and Baltimore, D. 1993. Regulation of the NFkappa B/rel transcription factor and I kappa B inhibitor system. Curr. Op. Cell Biol. 5:477–487.
Bours, V., Franzoso, G., Azarenko, V., Park, S., Kanno, T., Brown, K., and Siebenlist, U. 1993. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72:729–739.
Ghosh, S. and Baltimore, D. 1990. Activation in vitro of NFkappa B by phosphorylation of its inhibitor, I kappa B. Nature 344:678–682.
Link, E., Kerr, L., Schreck, R., Zabel, U., Verma, I., and Baeuerle, P. 1992. Purified I kappa B beta is inactivated upon dephosphorylation. J. Biol. Chem. 267:239–246.
Whiteside, S., Epinat, J., Rice, N., and Israel, A. 1997. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel activity. EMBO J. 16:1413–1426.
Inoue, J., Kerr, L., Kakizuka, A., and Verma, I. 1992. I kappa B gamma, a 70 kd protein identical to the c-terminal half of p110 NF-kappa B: A new member of the I kappa B family. Cell 68:1109–1120.
Beg, A., Ruben, S., Scheinman, R., Haskill, S., Rosen, C., and Baldwin, A. J. 1992. I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 6:1899–1913.
Rice, N., MacKichen, M., and Israel, A. 1992. The precurser to NF-kappa B p50 has I kappa B-like functions. Cell 71:243–253.
Chen, Z., Parent, L., and Maniatis, T. 1996. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination dependent protein kinase activity. Cell 84:853–862.
Mercurio, F., Zhu, H., Murray, B., Shevchenko, A., Bennet, B., Li, J., Young, D., Barbosa, M., Mann, M., and Rao, A. 1997. IKK-1 and IKK-2: Cytokine-activated I kappa B kinases essential for NF-kappa B activation. Science 278:860–866.
Woronicz, J., Gao, X., Cao, Z., Rothe, M., and Goeddel, D. 1997. I kappa B kinase-beta: NF-kappa B activation and complex formation with I kappa B kinase-alpha and NIK. Science 278:866–869.
Zandi, E., Chen, Y., and Karin, M. 1998. Direct phosphorylation of I kappa B by IKK-alpha and IKK-beta: discrimination between free and NF-kappa B bound substrate. Science 281:1360–1363.
Beg, A., Finco, T., Nantermet, P., and Jr., B. A. 1993. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol. Cell. Biol. 13:3310–3310.
Mellitis, K., Hay, R., and Goodbourn, S. 1993. Proteolytic degradation of MAD-3 (I kappa B alpha) and enhanced processing of the NF kappa B precursor p105 are obligatory steps in activation of NF-kappa B. Nucleic Acids Res. 21:5059–5066.
Yaron, A., Hatzubai, A., Davis, M., Lavon, I., Amit, S., Manning, A., Anderson, J., Mann, M., Mercurio, F., and Ben-Neriah, Y. 1998. Identification of the receptor component of the I kappa B alpha-ubiquitin ligase. Nature 396:590–594.
Palombella, V., Rando, O., Golberg, A., and Maniatis, T. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773–785.
Didonato, J., Mercurio, F., and Karin, M. 1995. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol. Cell. Biol. 15:1302–1311.
Torgerson, T., Colosia, A., Donahue, J., Lin, Y.-Z., and Hawiger, J. 1998. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear transport in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J. Immunol. 161:6084–6092.
Muller, J., Ziegler-Heitbrock, H., and Baeuerle, P. 1993. Nuclear Factor kappa B, a mediator or Lipopolysaccaride Effects. Immunobiol. 187:233–256.
Beg, A. A. and Baltimore, D. 1996. An essential role for NFkB in preventing TNF-a-induced cell death. Science. 274:782–784.
Van Antwerp, D. J., Martin, S. J., Kafri, T., Green, D., and Verma, I. M. 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787–789.
Wu, M., Lee, H., Bellas, R., Schauer, S., Arsura, M., Katz, D., Fitzgerald, M., Rothstein, T., Sherr, D., and Sonenshein, G. 1996. Inhibition of NF-kappa B/Rel induces apoptosis of murine B cells. EMBO J. 15:4682–4690.
Lezoualc'h, F., Sagara, Y., Holsboer, F., and Behl, C. 1998. High Constitutive NF-kappa B Activity Mediates Resistance to Oxidative Stress in Neuronal Cells. J. Neurosci. 18:3224–3232.
Clemens, J., Stephenson, D., Yin, T., Smalstig, E., Panetta, J., and Little, S. 1998. Drug-induced neuroprotection from global ischemia is assosiated with prevention of persistent but not transient activation of Nuclear Factor kappa B in rats. Stroke 29:677–682.
Wu, H. and Lozano, G. 1994. NF-kappa B Activation of p53. J. Biol. Chem. 269:20067–20074.
Wang, C.-Y., Mayo, M., Korneluk, R., Goeddel, D., and Baldwin, A. 1998. NF-kappa B Antiapoptosis: Induction of TRAF1 and TRAF2 and cIAP1 and cIAP2 to Supress Caspase-8 Activation. Science 281:1680–1683.
Wu, M., Ao, Z., Prasad, K., Wu, R., and Schlossman, S. 1998. IEX-1L, an Apoptosis Inhibitor Involved in NF-kappa BMediated Cell Survival. Science 281:998–1001.
Foo, S. and Nolan, G. 1999. NF-kappa B to the rescue; RELs, apoptosis, and cellular transformation. TIG 15:229–235.
Lee, H., Dadgostar, H., Cheng, Q., Shu, J., and Cheng, G. 1999. NF-kappa B mediated up-regulation of Bcl-x and Bfl-I/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 96:9136–9141.
Glasgow, J. N., Wood, T., and Perez-Polo 2000. Identification and characterization of NF-κB binding sites in the murine bcl-x promoter. J. Neurochem. 75:1377–1389.
Hettmann, T., Didonato, J., Karin, M., and Leiden, J. 1999. An Essential Role for NF-kappa B in Promoting Double Positive Thymocyte Apoptosis. J. Exp. Med. 189:145–157.
Tamatani, M., Che, Y., Matsuzaki, H., Ogawa, S., Okado, H., Miyake, S., Mizuno, T., and Tohyama, M. 1999. Tumor Necrosis Factor Induces Bcl-2 and Bcl-x Expression through NF-kappa B Activation in Primary Hippocampal Neurons. J. Biol. Chem. 274:8531–8538.
Dixon, E., Stephenson, D., Clemens, J., and Little, S. 1997. Bcl-Xshort is elevated following severe global ischemia in rat brains. Brain Res. 776:222–229.
Saikumar, P., Dong, Z., Weinberg, J. M., and Venkatachalam, M. A. 1998. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 17:3341–3349.
Scott, R. J. and Hegyi, L. 1997. Cell death in perinatal hypoxicischaemic brain injury. Neuropath. Appl. Neurobiol. 23:307–314.
Pulera, M. R., Adams, L. M., Liu, H., Santos, D. G., Nishimura, R. N., Yang F., Cole G. M., and Wasterlain, C. G. 1998. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 29:2622–2630.
Charriaut-Marlangue, C., Margaill, I., Represa, A., Popovici, T., Plotkine, M., and Ben-Ari, Y., 1996. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J. Cereb. Blood Flow Metab. 16:186–194.
Piantadosi, C. A., Zhang, J., and Demchenko, I. T. 1997. Production of hydroxyl radical in the hippocampus after CO hypoxia or hypoxic hypoxia in the rat. Free Rad. Biol. Med. 22:725–732.
Schmidt-Kastner, R., Fliss, H., and Hakim, A. M. 1997. Subtle neuronal death in striatum after short forebrain ischemia in rats detected by in situ end labeling for DNA damage. Stroke 28:163–170.
Kent, T. A., Quast, M., Taglialatela, G., Rea, C., Wei, J., Tao, Z., and Perez-Polo, J. R. 1999. Effect of NGF treatment on outcome measures in a rat model of middle cerebral artery occlusion. J. Neurosci. Res. 55:357–369.
Lin, Y. and Greysen, G. 1996. Analysis of the risk of brain damage in asphyxiated infants. J. Perinat. Med. 24:581–589.
Naulty, C. M., Long, L. B., and Pettett G. 1994. Prevalence of prematuarity low birthweight, and asphyxia as perinatal risk factors in a current population of children with cerebral palsy. Am. J. Perinatol. 11:377–381.
Patel, J. and Edwards, A. D. 1997. Prediction of outcome after perinatal asphyxia. Curr. Opin. Pediatr. 9:128–132.
Volpe, J. J. 1995. Hypoxic-ischemic encephalogpathy: intrauterine assessment. In: Neurology of the newborn. Pp260–278. WBSaunders Co. Philadelphia.
Seidl, R., Stockler-Ipsiroglu, S., Rolinski, B., Kohlhauser, C., Herner, K. R., Lubec, B., and Lubec, G. 2000. Energy metabolism in graded perinatal asphyxia of the rat. Life Sci. 67:421–435.
Cooper, C. E. 1999. In vivo measurements of mitochondiral function and cell death following hypoxic/ischaemic damage to the new-born brain. Biochem. Soc. Symp. 66:123–140.
Clawson, T. F., Vannucci, S. J., Wang, M. G., Seaman, L. B., Yang, X. L., and Lee, W. H. 1999. Hypoxia-ischemia-induced apoptotic cell death correlates with IGF-I mRNA decrease in neonatal rat brain. Biol. Sig. Rec. 8:281–293.
Vannucci, R. C., Connor, J. R., Mauger, D. T., Palmer, C., Smith, M. B., Towfighi, J., and Vannucci, S. J. 1999. Rat model of perinatal hypoxic-ischemic brain damage. [Review] J. Neurosci. Res. 55:158–163.
Barth, A., Bauer, R., Gedrange, T., Walter, B., Klinger, W., and Zwiener, U. 1998. Influence of hypoxia and hypoxia/hypercapnia upon brain and blood peroxidative and glutathione status in normal weight and growth-restricted newborn piglets. Exp. Tox. Path. 50:402–410.
Nedelcu, J., Klein, M. A., Aguzzi, A., Boesiger, P., and Martin, E. 1999. Biphasic edema after hypoxic-ischemic brain injury in neonatal rats reflects early neuronal and late glial damage. Pediatr. Res. 46:297–304.
Barkovich, A. J., Baranski, K., Vigneron, D., Partridge, J. C., Hallam, D. K., Hajnal, B. L., and Ferriero, D. M. 1999. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. Am. J. Neurorad. 20:1399–1405.
Thornton, J. S., Ordidge, R. J., Penrice, J., Cady, E. B., Amess, P. N., Punwani, S., Clemence, M., and Wyatt, J. S. 1998. Temporal and anatomical variations of brain water apparent diffusion coeeficient in perinatal cerebral hypoxic-ischemic injury: relationships to cerebral energy metabolism. Mag. Res. Med. 39:920–927.
Delivoria-Papadopoulos, M. and Mishra, O. P. 1998. Mechanisms of cerebral injury in perinatal asphyxia and strategies for prevention. [Review] J. Pediatr. 132:S30–34.
Wang, Z. F., Xue, C. S., Zhou, Q. X., Wan, Z. B., and Luo, Q. S. 1999. Effects of tetrandrine on changes of NMDA receptor channel in cortical neurons of rat induced by anoxia. Zhongguo Yao Li Xue Bao 20:729–732.
Volpe, J. J. 1998. Neurologic outcome of prematurity. Arch Neurol. 55:297–300.
Carro, E., Senaris, R. M., Mallo, F., and Dieguez, C. 1998. Inhibin suppresses in vivo growth hormone secretion. Neuroendocrinol. 68:293–296.
Gozal, E., Simakajomboon, N., and Gozal, D. 1998. NF-KB induction during in vivo hypoxia in dorsocaudal brain stem of rateffect of MK-801 and L-NAME. J. Appl. Physiol. 85:372–376.
Carter, B. D., Kaltschmidt, C., Kaltschmidt, B., Offenhauser, N., Böhm-Matthaei, R., Baeuerle, P., and Barde, Y.-A. 1996. Selective activation of NF B by nerve growth factor through the neurotrophin receptor p75. Science 272:542–545.
Taglialatela, G., Kauffman, J. A., Trevino, A., and Perez-Polo, J. R. 1998. Central Nervous System DNA fragmentation induced by the inhibition of nuclear factor Kappa B. NeuroReport 9:489–493.
Flohe, L., Brigelius-Flohe, R., Saliou, C. Traber, M. G., and Packer, L. 1997. Redox Regulation of NF-Kappa B action. Free Rad. Biol. Med. 22:1115–1126.
Zhang, S., Tobaru, T., Zivin, J. A., and Shackelford, D. A. 1998. Activaiton of nuclear factor-κB in the rabbit spinal cord followingischemia and reperfusion. Mol. Brain Res. 63:121–132.
Franzoso, G., Bours, V., Azarenko, V., Park, S., Tomita-Yamaguchi, M., Kanno, T., Brown, K., and Siebenlist, U. 1993. The oncoprotein Bcl-3 can facilitate NF-kappa B-mediated transactivation by inhibiting p50 homodimers from select kappa B sites. EMBO J. 12:3893–3901.
Fujita, T., Nolan, G. P., Liou, H. C., Scott, M. L., and Baltimore, D. 1993. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that acts through NF-kappa B p 50 homodimers. Genes Dev. 7:1354–1363.
Nolan, G. P., Fujita, K., Huppi, C., Liou, H. C., Scott, M. L., and Baltimore, D. 1993. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that protects and interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol. Cell Biol. 13:3557–3566.
Bundy, D. L. and McKeithan, T. W. 1997. Diverse effects of Bcl 3 phosphorylation on its modulation of NF-kappaB p50 homodimer binding to DNA. J. Biol. Chem. 272:33132–33139.
Bohuslav, J., Kravchenko, V. V., Parry, G. C., Erlich, J. H., Gerondakis, S., Mackman, N., and Ulevitch, R. J. 1998. J. Clin. Invest. 102:1645–1652.
Watanabe, N., Iwamura, T., Shinoda, T., and Fujita, T. 1997. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generated kappaB homodimers from the cytoplasmic pool of p50–p105 and nuclear transcription factor. EMBO J. 16:3609–3620.
Na, S. Y., Choi, J. E., Kim, H. J., Jhun, B. H., Lee, Y. C., and Lee, J. W. 1999. J. Biol. Chem. 274:28491–28496.
Siebenlist, U., Frazoso, G., and Brown, K. 1994. Structure, regulation and function of NF-κB. Ann. Rev. Cell Bio. 10:405–455.
Heissmeyer, V., Krappmann, D., Wulczyn, F. G., and Scheidereit, C. 1999. NF-kappaB p105 is a target of IkappaB kinases and controls signal induction of p50 complexes. EMBO J. 18:4766–4778.
Rebollo, A., Dumoutier, L., Renauld, J. C., Zaballos, A., Ayllon, V., and Martinez, A. C. 2000. Bcl-3 expression promotes cell survival following interleukin-4 deprivation controlled by AP1 and AP1-like transcription factors. Mol. Cell Biol. 20:3407–3416.
Isenmann, S., Stoll, G., Schroeter, M., Krajewski, S., Reed, J. C., and Baerh, M. 1998. Differential regulation of Bax, Bcl-2, and Bcl-x proteins in focal cortical ischemia in the rat. Br. Pathol. 8:49–62.
Parsadanian, A. S., Cheng, Y., Keller-Peck, C. R., Holtzman, D. M., and Snider, W. D. 1998. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J. Neurosci. 18:1009–1019.
Lou, J., Lenke, L. G., Xu, F., and O'Brien, M. 1998. In vivo Bcl-2 oncogene neuronal expression in the rat spinal cord. Spine 23:517–523.
Martinou, J. C., Dubois-dauphin, M., Staple, J. K., Rodriguez, I., Frankowski, H., Roth, K. A., Motoyama, N., and Loh, D. Y. 1996. Apoptosis of Bcl-xL deficient telencephalic cells in vitro. J. Neurosci. 16:1753–1758.
Li, G. L., Brodin, G., Farooque, M., Funa, K., Holtz, A., Wang, W. L., and Olsson, Y. 1996. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J. Neuropath. & Exp. Neurol. 55:280–289.
Vogt, M., Bauer, M. K., Ferrari, D., and Schulze-Ostroff, K. 1998. Oxidative stress and hypoxia/reoxygenation trigger CD95 (APO-1/Fas) ligand expression in microglial cells. FEBS Lett. 429:67–72.
Ichiyama, T., Zhao, H., Catania, A., Furukawa, S., and Lipton, J. M. 1999. Alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation and IkappaBalpha degradation in human glioma cells and experimental brain inflammation. Exp. Neur. 157:359–365.
Kessler, J. A., Ludlam, W. H., Freidin, M. M., Hall, D. H., Michaelson, M. D., Spray, D. C., Dougherty, M., and Batter, D. K. 1993. Cytokine-induced programmed cell death of cultured sympathetic neurons. Neuron 11:1123–1132.
Kitajima, I., Soejima, Y., Taksaki, I., Beppu, H., Tokioka, T., and Maruyama, I. 1996. Ceramide-induced nuclear translocation of NF-κB is a potential mediator of the apoptotic response to TNF-α in murine clonal osteoblasts. Bone 19:263–270.
Lin, K. I., Lee, S. H., Narayanan, R., Baraban, J. R., Hardwick, J. M., and Ratan, R. R. 1995. Thiol agents and bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of NF-κB. J. Cell Biol. 131:1–14.
Grilli, M., Pizzi, M., Memo, M., and Spano, P. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 274:1383–1385.
Clemens, J. A., Stephenson, D. T., Smalstig, E. B., Dixon, E. P., and Little, S. P. 1997. Global ischemia activates nuclear factor kappa B in forebrain neurons of rats. Stroke 28:1073–1080.
Barger, S. W., Horster, D., Furukawa, K., Goodman, Y., Krieglstein, J., and Mattson, M. P. 1995. Tumor necrosis factors α and β protect neurons against amyloid β-peptide toxicity: evidence for involvement of a kB-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc. Natl. Acad. Sci. USA 92:9238–9332.
Barger, S. W. and Mattson, M. P. 1996. Induction of neuroprotective kappa B-dependent transcription by secreted forms of the Alzheimer's beta-amyloid precursor. Mol. Brain Res., 40:116–126.
Goodman, Y. and Mattson, M. P. 1996. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults and amyloid β-peptide toxicity. N. Neurochem. 66:869–872.
Grilli, M. and Memo, M. 1999. Nuclear factor kappa B/Rel proteins: a point of convergence of signaling pathways relevant in neuronal function and dysfunction. Biochem. Pharmacol. 57:1–7.
Kitamura, Y., Shimohama, S., Kamoshima, W., Ota, T., Matsuoka, Y., Nomura, Y., Smith, M. A., Perry, G., Whitehouse P. J., and Taniguchi, T. 1995. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer's disease. Brain Res. 780:260–269, 1998.
Boise, H., Gottschalk, A. R., Quintans, J., and Thompson, C. B. Bcl-2 and Bcl-2-related proteins in apoptosis regulation. Curr. Top. Microbiol. Immunol. 200:107–121.
Bredesen, D. 1995. Neural apoptosis. Anal. Neurol. 38:839–851.
Hockenbery, D. M., Oltvai, Z. N., Yin, X.-M., Milliman, C. L., and Korsmeyer, S. J. 1993. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241–251.
Kiefer, M. C., Brauer, M. J., Powers, V. C., Wu, J. J., Umansky, S. R., Tomei, L. D., and Barr, P. J. 1995. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 374:736–739.
Krajewski, S., Karjewska, M., and Reed, J. C. 1996. Immunohistochemical analysis of in vivo patterns of Bak expression, a pro-apoptotic member of the Bcl-2 family. Cancer Res. 56:2849–2855.
Su, J. H., Satou, T., Anderson, A. J., and Cotman, C. W. 1996. Up-regulation of Bcl-2 is associated with neuronal DNA damage in Alzheimer's disease. NeuroReport 7:437–440.
Su, J. H., Deng, G., and Cotman, C. W. 1997. Bax protein expression is increased in Alzheimer's brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J. Neuropath. Exp. Neurol. 56:86–93.
Maroto, R. and Perez-Polo, J. R. 1997. BCL-2 protein expression in apoptosis: oxidative stress versus serum deprivation in PC12 cells. J. Neurochem. 69:514–523.
Chen, J., Zhu, R. L., Nakayama, M., Kawaguchi, K., Jin, K., Stetler, R. A., Simon, R. P., and Graham, S. H. 1996. Expression of the apoptosis-effector gene, Bax, is up-regulated in vulnerable hippocampal CA1 neurons following global ischemia. J. Neurochem. 67:64–71.
Hara, A., Iwai, T., Niwa, M., Uematsu, T., Yoshimi, N., Tanaka,T., and Mori, H. 1996. Immunohistochemical detection of Bax and Bcl-2 proteins in gerbil hippocampus following transient forebrain ischemia. Br. Res. 711:249–253.
Antonawich, F. J., Krajewski, S., Reed, J. C., and Davis, J. N., 1998. Bcl-x1 Bax interaction after transient global ischemia. J. Cereb. Blood Flow Metab. 18:882–886.
Niwa, M., Hara A., Iwai, T., Sassa, T., Mori, H., and Uematsu, T. 1997. Expression of Bax and Bcl-2 protein in the gerbil hippocampus following transient forebrain ischemia and its modification by phencyclidine. Neurol. Res. 19:629–633.
Gillardon, F., Lenz, C., Waschke, K. F., Krajewski, S., Reed, J. C., Zimmermann, M., and Kuschinsky, W. 1996. Altered expression of Bcl-2, Bcl-X, Bax, and c-Fos colocalizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Mol. Br. Res. 40:254–260.
Honkaniemi, J., Massa, S. M., Breckinridge, M., and Sharp, F. R. 1996. Global ischemia induces apoptosis-associated genes in hippocampus. Mol. Br. Res. 42:79–88.
Ferrer, I., Lopez, E., Blanco, R., Rivera, R., Ballabriga, J., Pozas, E., and Martie, E. 1998. Bcl-2, Bax, and Bcl-x expression in the CA1 area of the hippocampus following transient forebrain ischemia in the adult gerbil. Exp. Brain Res. 121:167–173.
Kessler, J. A., Ludlam, W. H., Freidin, M. M., Hall, D. H., Michaelson, M. D., Spray, D. C., Dougherty, M., and Batter, D. K. 1993. Cytokine-induced programmed cell death of cultured sympathetic neurons. Neuron 11:1123–1132.
Wen, X., Furhman, S., Michaels, G. S., Carr, D. B., Smith, S., Barker, J. L., and Somogyi, R. 1998. Large-scale temporal gene expression mapping of central nervous system development. Proc. Natl. Acad. Sci. USA 95:334–339.
Yu, Z., Zhou, D., Bruce-Keller, A. J., Kindy, M. S., and Mattson, M. P. 1999. Lack of the p50 subunit of nuclear factorkappa B increases the vulnerability of hippocampal neurons to excitotoxic injury. J. Neurosci. 19:8856–8865.
Abbadie, C., Kabrun, N., Bouali, F., Smardova, J., Stehelin, D., Vandenbunder, B., and Enrietto, P. J. 1993. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell 75:899–912.
Jung, S., Yaron, A., Alkalay, I., Hatzubai, A., Avraham, A., and Ben-Neriah, Y., 1995. Costimulation requirement for AP-1 and NF-kappa B transcription factor activation in T cells. Ann. NY Acad. Sci. 766:245–252, 1995.
Grilli, M., Pizzi, M., Memo, M., and Spano, P. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 274:1383–1385.
Grimm, S., Bauer, M. K. A., Baeuerle, P. A., and Schulze-Osthoff, K. 1996. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J. Cell Biol. 134:13–23.
Baichwal, V. R. and Baeuerle, P. A. 1997. Activate NF-kappa B or die? Curr. Biol. 7:R94–96.
Lipton, S. A. 1997. Janus faces of NF-kappa B: neurodestruction versus neuroprotection. Nat. Med. 3:20–22.
Qiu, J.-X., Glasgow, J., Kent, T. A., Rassin, D. K., and Perez-Polo, J. R. 2001. Differential NF-κB regulation of bcl-x gene expression in Hippocampus and basal forebrain in response to hypoxia. J. Neurosci. Res. 64:223–234.
Vaux, D. L. 1993. Toward understanding of the molecular mechanisms of physiological cell death. Proc. Nat. Acad. Sci. USA 90:786–789.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glasgow, J.N., Qiu, J., Rassin, D. et al. Transcriptional Regulation of the BCL-X Gene by NF-κB Is an Element of Hypoxic Responses in the Rat Brain. Neurochem Res 26, 647–659 (2001). https://doi.org/10.1023/A:1010987220034
Issue Date:
DOI: https://doi.org/10.1023/A:1010987220034