Abstract
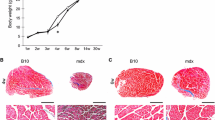
X chromosome-linked muscular dystrophic mdx mouse lacks the sarcolemmal protein dystrophin and represents a genetic homologue of human Duchenne muscular dystrophy (DMD). The present study analysed some aspects of pathological processes such as fibrosis, frequency of centralized nuclei, presence of degenerative or regenerative fibres, expression of utrophin and associated protein complexes, and myosin heavy chain isoforms in three muscles [diaphragm (DIA), gastrocnemius (GTC) and masseter (MAS)] from old male mdx mice. All parameters investigated comparatively in these pathological muscles provided evidence that the MAS mdx muscle presents a slight deterioration pattern in comparison to that of DIA and GTC muscles. Utrophin and associated proteins are present in many cell clusters with continuous membrane labelling in MAS muscle. Respective proportions of myosin heavy chain isoforms, measured by electrophoresis/densitometry, showed only slight change in GTC muscle, significant evolution in DIA muscle but drastic isoform conversions in MAS muscle. These results highlighted the difference in deterioration susceptibility of various muscles to muscular dystrophy. The reason why this occurs in MAS muscles is still obscure and discussed in terms of the comparative developmental origins of these muscles.
Similar content being viewed by others
References
Bobet J, Mooney RF and Gordon T (1998) Force and stiffness of old dystrophic (mdx) mouse skeletal muscles. Muscle Nerve 21: 536-539.
Boland B, Himpens B, Denef JF and Gillis JM (1995) Site-dependent pathological differences in smooth muscles and skeletal muscles of the adult mdx mouse. Muscle Nerve 18: 649-657.
Blough ER, Rennie ER, Zhang F and Reiser PJ (1996) Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem 233: 31-35.
Brussee V, Tardif F and Tremblay JP (1997) Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromusc Disord 7: 487-492.
Carnwath JW and Shotton DM (1987) Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci 80: 39-54.
Carter GT, Abresch RT, Walsh SA and Wineinger MA (1997) The mdx mouse diaphragm: exercise-induced injury. Muscle Nerve 20: 393-394.
Chaubourt E, Fossier P, Baux G, Leprince C, Israel M and De La Porte S (1999) Nitric oxide and L-arginine cause an accumulation of utrophin at the sarcolemma: a possible compensation for dystrophin loss in Duchenne muscular dystrophy. Neurobiol Disease 6: 499-507.
Chaubourt E, Voisin V, Fossier P, Baux G, Israel M and De La Porte S (2000) The NO way to increase muscular utrophin expression? C R Acad Sci Life Sci 323: 735-740.
Clerk A, Morris GE, Dubowitz V, Davies KE, and Sewry CA (1993) Dystrophin-related protein, utrophin, in normal and dystrophic human fetal skeletal muscle. Histochem J 25: 554-561.
Coulton GR, Morgan JE, Partridge TA and Sloper JC (1988) The mdx mouse skeletal muscle myopathy. I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14: 53-70.
D'Albis A, Couteaux R, Janmot C and Roulet A (1989) Specific programs of myosin expression in the post-natal development of rat muscles. Eur J Biochem 183: 583-590.
Dangain J and Vrbova G (1984) Muscle development in mdx mutant mice. Muscle Nerve 7: 700-704.
Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies KE and Gillis JM (1997) Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nature Medicine 3: 1216-1221.
Deconinck N, Rafael JA, Beckers-Bleukx G, Kahn D, Deconinck AE, Davies KE and Gillis JM (1998) Consequences of the combined deficiency in dystrophin and utrophin on the mechanical properties and myosin composition of limb and respiratory muscles of the mouse. Neuromuscul Disord 8: 362-370.
Dick J and Vrbova G (1993) Progressive deterioration of muscles in mdx mice induced by overload. Clin Sci 84: 145-150.
DiMario JX, Uzman A and Strohman RC (1991) Fiber regeneration is not persistent in dystrophic (mdx) mouse skeletal muscle. Dev Biol 148: 314-321.
Dupont-Versteegden EE and McCarter RJ (1992) Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve 15: 1105-1110.
Dupont-Versteegden EE, McCarter RJ and Katz MS (1994) Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol 77: 1736-1741.
Geissinger HD, Prasada Rao PVV and McDonald-Taylor CK (1990) 'mdx’ mouse myopathy: histopathological, morphometric and histochemical observations on young mice. J Comp Path 102: 249-263.
Hayes A, Lynch GS and Williams DA (1993) The effects of endurance exercise on dystrophic mdx mice. Proc R Soc Lond B 253: 19-25.
Hayes A and Williams DA (1998) Contractile function and low-intensity exercise effects of old dystrophic (mdx) mice. Am J Physiol 43: C1138-C1144.
Helliwell TR, MacLennan PA, McArdle A, Edwards RHT and Jackson MJ (1996). Fasting increases the extent of muscle necrosis in the mdx mouse. Clin Sci 90: 467-472.
Hoffman EP, Brown RH and Kunkel LM (1987) Dystrophin: the protein product of Duchenne muscular dystrophy locus. Cell 51: 919-928.
Iannaccone S, Quattrini A, Smirne S, Sessa M, de Rino F, Ferini-Strambi L and Nemni R (1995) Connective tissue proliferation and growth factors in animal models of Duchenne muscular dystrophy. J Neurol Sci 128: 36-44.
Ibebunjo C, Srikant CB and Donati F (1996) Properties of fibres, endplate and acetylcholine receptors in the diaphragm, masseter, laryngeal, abdominal and limb muscle in the goat. Can J Anaesth 43: 475-484.
Karpati G and Carpenter S (1986) Small-caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am J Med Genet 25: 653-658.
Karpati G, Carpenter S and Prescott S (1988) Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle Nerve 11: 795-803.
Khurana TS, Prendergast RA, Alameddine HS, Tome FM, Fardeau M, Arahata K, Sugita H and Kunkel LM (1995) Absence of extraocular muscle pathology in Duchenne's muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med 182: 467-475.
Louboutin JP, Fichter-Gagnepain V, Thaon E and Fardeau M (1993) Morphometric analysis of mdx diaphragm muscle fibres. comparison with hindlimb muscles. Neuromusc Disord 3: 463-469.
Marcello N, Ortaggio F and Sabadini R (1994) The masticatory function in DMD patients by video-tape investigations. Acta Cardiomiologica 1: 69-78.
Marcucio RS and Noden DM (1999) Myotube heterogeneity in developing chick craniofacial skeletal muscles. Dev Dynamics 214: 178-194.
Matsuda R, Nishikawa A and Tanaka H (1995) Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem 118: 959-964.
McArdle A, Edwards RHT and Jackson MT (1995) How does dystrophin deficiency lead to muscle degeneration? Evidence from the mdx mouse. Neuromusc Disord 5: 445-456.
McCully KK (1986) Exercise-induced injury to skeletal muscle. Fed Proc 45: 2933-2936.
Moens P, Baatsen PHW and Marechal G (1993) Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14: 446-451.
Muller J, Vayssiere N, Muller A, Marti-Mestres G and Mornet D (2000) Bilateral effect of a unilateral occlusal splint on the expression of myosin heavy-chain isoforms in rat deep masseter muscle. Arch Oral Biol 45: 1017-1024.
Nonaka I (1998) Animal models of muscular dystrophies. Lab Anim Sci 48: 8-17.
Pastoret C and Sebille A (1993) Further aspects of muscular dystrophy in mdx mice. Neuromusc Disord 3: 471-475.
Pastoret C and Sebille A (1995) Mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci 129: 97-105.
Petrof BJ, Stedman HH, Shrager JB, Eby J, Sweeney HL and Kelly AM (1993) Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol 265: C834-C841.
Pons F, Robert A, Marini JF and Leger JJ (1994) Does utrophin expression in muscle of mdx mice functionally compensate for dystrophin deficiency during postnatal development? J Neurol Sci 122: 162-170.
Porter JD and Baker RS (1996) Muscles of a different ‘color’: the unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic disease. Neurology 46: 30-37.
Porter JD, Rafael JA, Ragusa RJ, Brueckner JK, Trickett JI and Davies KE (1998) The sparing of extraocular muscle in dystrophinopathy is lost in mice lacking utrophin and dystrophin. J Cell Sci 111: 1801-1811.
Porter JD (1998) Commentary: extraocular muscle sparing in muscular dystrophy: a critical evaluation of potential protective mechanisms. Neuromusc Disord 8: 198-203.
Ragusa RJ, Chow CK, St Clair DK and Porter JD (1996) Extraocular, limb and diaphragm muscle group-specific antioxidant enzyme activity patterns in control and mdx mice. J Neurol Sci 139: 180-186.
Ragusa RJ, Chow CK and Porter JD (1997) Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromusc Disord 7: 379-386.
Rafael JA, Townsend ER, Squire SE, Potter AC, Chamberlain JS and Davies KE (2000) Dystrophin and utrophin influence fibre type composition and post-synaptic membrane structure. Human Mol Genet 9: 1357-1367.
Reggiani C, Brocks L, Wirtz P, Loermans H and Kronnie G (1992) Myosin isoforms in hindlimb muscles of normal and dystrophic (ReJ129 dy/dy) mice. Muscle Nerve 14: 199-208.
Rowlerson AM (1990) Specialization of mammalian jaw muscles: fibre type compositions and the distribution of muscle spindles. In: Taylor A (eds) Neurophysiology of the Jaws and Teeth. Macmillan: London, 1-51.
Sacco P, Jones DA, Dick JR and Vrbova G (1992) Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci 82: 227-236.
Schiaffino S and Reggiani C (1994) Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77: 493-501.
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG and Barnard PS (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578-1580.
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT and Kelly AM (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352: 536-539.
Straub V, Rafael JA, Chamberlain JS and Campbell KP (1997) Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol 139: 375-385.
Talmadge RJ and Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337-2340.
Tanabe Y, Esaki K and Nomura T (1986) Skeletal muscle pathology in X-chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol 69: 91-95.
Taylor J, Muntoni F, Dubowitz V and Sewry CA (1997) The abnormal expression of utrophin in Duchenne and Becker muscular dystrophy is age related. Neuropathol Appl Neurobiol 23: 399-405.
Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM and Davies K (1998) Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nature Medicine 4: 141-144.
Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M and Tremblay JP (1998) Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve 21: 567-576.
Webster C, Silberstein L, Hays AP and Blau HM (1988) Fast muscle fibres are preferentially affected in Duchenne muscular dystrophy. Cell 52: 503-513.
Weller B, Karpati G and Carpenter S (1990) Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci 100: 9-13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muller, J., Vayssiere, N., Royuela, M. et al. Comparative evolution of muscular dystrophy in diaphragm, gastrocnemius and masseter muscles from old male mdx mice. J Muscle Res Cell Motil 22, 133–139 (2001). https://doi.org/10.1023/A:1010305801236
Issue Date:
DOI: https://doi.org/10.1023/A:1010305801236