Abstract
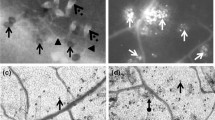
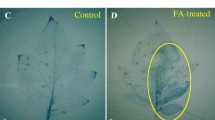
Application of o-hydroxyethylorutin restricted the development of Botrytis cinerea in tomato leaves. Superoxide anion and hydrogen peroxide generation rates and changes in superoxide dismutase, peroxidase and catalase activities were studied in uninfected tomato plants, in plants infected with B. cinerea, and in plants treated with o-hydroxyethylorutin and infected with pathogen. About two times higher hydrogen peroxide concentration were found in plants treated with o-hydroxyethylorutin and infected with the pathogen at the early infection stages compared with untreated infected plants. In vitro tests showed that germination of B. cinerea conidia was significantly inhibited by H2O2. Higher H2O2 concentrations were needed to inhibit mycelial growth. The results indicate that o-hydroxyethylorutin triggers hydrogen peroxide production in tomato plants and suggest that enhanced levels of H2O2 are involved in restricted B. cinerea infection development.
Similar content being viewed by others
References
Abeles FB, Dunn LJ, Morgens P, Callaham A, Dinterman RE and Schmidt J (1988) Induction of 33-kD and 60-kD peroxidases during ethylene-induced senescence of cucumber cotyledones. Plant Physiol 87: 609-615
Ádám AL, Bestwick CS, Barna B and Mansfield JW (1995) Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. phaseolicola. Planta 197: 240-249
Alscher RG, Donahue JL and Cramer CL (1997) Reactive oxygen species and antioxidants: relationship in green cells. Physiol Plant 100: 224-233
Baker CJ and Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33: 299-321
Baker CJ, Harmon GL, Glazener JA and Orlandi EW (1995) A noninvasive technique for monitoring peroxidative and H2O2-scavenging activities during interactions between bacterial plant pathogens and suspension cells. Plant Physiol 108: 353-359
Bartoli CG, Simontacchi M, Guiamet JG, Montaldi E and Puntarulo S (1995) Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci 104: 161-168
Beauchamp C and Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem 44: 276-287
Bestwick CHS, Brown IR and Mansfield JW (1998) Localised changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive rection in lettuce. Plant Physiol 118: 1067-1078
Bolwell GP and Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant-a broad perspective. Physiol Mol Plant Pathol 51: 347-366
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254
Bruneton J (1995) Flavonoids. In: Pharmacognosy, Phytochemistry Medicinal Plants (pp 265-301) Lavoisier Pub Inc Springer-Verlag, Paris
Cao G, Sofic E and Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationship. Free Radical Biol Med 22(5): 749-760
Capaldi DJ and Taylor KE (1983) A new peroxidase colour reaction: oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with its formaldehyde azine application to glucose and choline oxidases. Analyt Biochem 129: 329-336
Chen Z, Silva H and Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883-1886
Cohen Y (1994) 3-Aminobutyric acid induces systemic resistance against Peronospora tabacina. Physiol Mol Plant Pathol 44: 237-288
Conrath U, Chen Z, Ricigliano JR and Klessig DF (1995) Two inducers of plant defense responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA 92: 7143-7147
Dhindsa RS, Plumb-Dhindsa P and Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decrease levels of superoxide dismutase and catalase. J Exp Bot 32: 93-101
Dixon RA and Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7: 1085-1097
Doke N (1983) Involvement of superoxide generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophtora infestans and to the hyphal wall components. Physiol Plant Pathol 23: 345-357
Glinka R, Żak E and Łęka E (1995) Newpharmaceutical and cosmeticall forms containing o-hydroxyethylorutine as the active substance. Zesz Nauk Politech Łódz 708: 69-76
Gould KS, Kuhn DN, Lee DW and Oberbauer ST (1995) Why leaves are sometimes red. Nature 378: 241-242
Lamb C and Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251-275
Low PS and Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96: 533-542
Lu H and Higgins VJ (1999) The effect of hydrogen peroxide on the viability of tomato cells and of the fungal pathogen Cladosporium fulvum. Physiol Mol Plant Pathol 54: 131-143
Maehly AC and Chance B (1954) The assay of catalase and peroxidases. In: Glick D. (ed) Methods of Biochemical Analysis, Vol 1 (pp 357-424) Interscience Publishers Inc, New York
Małolepsza U, Urbanek H, Pietras T and Glinka R (1998) The superoxide anion generation and enzymatic antioxidants activities in tomato leaves after o-hydroxyethylorutin treatment and fungal pathogen infection. Biul Kosmetol Poland 1: 44-47
Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105: 467-472
Mehdy MC, Sharma YK, Sathasivan K and Bays NW (1996) The role of activated oxygen species in plant disease resistance. Physiol Plant 98: 356-374
MilosevińN and Slusarenko AJ (1996) Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiol Mol Plant Pathol 49: 143-158
Miniati E and Montanari L (1998) Extraction and purification of rutin from tobacco leaf protein technology wastes. Ital J Food Sci 4: 339-349
Nakano Y and Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867-880
Ogawa K, Kanematsu S and Asada K (1997) Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol 38: 1118-1126
Okuda T, Matsuda Y, Yamanaka A and Sagisaka S (1991) Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97: 1265-1267
Panava T and Rubinstein B (1998) Oxidative events during programmed cell death of daylily (Hemerocalli hybrid) petals. Plant Sci 133: 125-132
Peng M and Kuć J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82: 696-699
Rice-Evans CA, Miller NJ and Paganga G (1997) Antioxidant properties of phenolic compounds. Trend Plant Sci 2: 152-159
Takahama U and Oniki T (1997) A peroxidase/phenolics/ ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol Plant 101: 845-852
Tenhaken R, Levine A, Brisson L, Dixon RA and Lamb CHF (1995) Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA 92: 4158-4163
Velikova V, Yordanov I and Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151: 59-66
Wendehenne D, Durner J, Chen Z and Klessig DF (1998) Benzothiadiazole, an inducer of plant defenses, inhibits catalase and ascorbate peroxidase. Phytochemistry 47: 651-657
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322: 681-692
Wu G, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons KC and Shah DM (1995) Disease resistance conferred by expression of a gene encodingH2O2-generating glucose oxidase in transgenic potato plants. Plant Cell 7: 1357-1368
Yamasaki H, Sakihama Y and Ikehara N (1997) Flavonoidperoxidase reaction as a detoxification mechanisms of plant cell against H2O2. Plant Physiol 115: 1405-1412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Małolepsza, U., Urbanek, H. The Oxidants and Antioxidant Enzymes in Tomato Leaves Treated With O-Hydroxyethylorutin and Infected with Botrytis cinerea. European Journal of Plant Pathology 106, 657–665 (2000). https://doi.org/10.1023/A:1008719820600
Issue Date:
DOI: https://doi.org/10.1023/A:1008719820600