Abstract
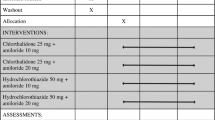
The antihypertensive effects and tolerance of once-daily barnidipine, a novel dihydropyridine calcium antagonist, were evaluated. A total of 190 patients with a sitting diastolic blood pressure (DBP) of 95–114 mmHg were investigated in this multicenter, double-blind, placebo-controlled, dose-ranging study. After a 4-week single-blind placeborun-in period, patients were randomized to placebo or barnidipine (10 mg, 20 mg, or 30 mg modified release capsules) once daily for 6 weeks. Nonresponders (sitting DBP ≥90 mmHg and a decrease of <10 mmHg) were treated for an additional 6 weeks with a dose increase of 10 mg. At each clinic visit, sitting and standing blood pressure and heart rate were measured approximately 24 hours after the last dose of study drug was taken. Compared with placebo, barnidipine lowered blood pressure, with a trend toward a dose–response relationship over the dose range 10–30 mg. A dose increment of 10 mg in nonresponders resulted in additional reductions in blood pressure. At the end of the active treatment period, the responder rates were 41% and 57% for 10 mg and 20 mg barnidipine, respectively. Heart rate in both sitting and standing positions was not affected by barnidipine. Treatment with barnidipine was well tolerated, and the incidence of adverse events was dose related and consistent with vasodilatation. In conclusion, barnidipine (10–30 mg) administered once daily is well tolerated and reduces blood pressure inpatients with mild to moderate hypertension.
Similar content being viewed by others
References
World Health Organization/International Society of Hypertension. 1993 Guidelines for the Management of Mild Hypertension: Memorandum from a World Health Organization/International Society of Hypertension meeting. J Hypertens 1993;11:905–918.
Satoh H. Pharmacology and therapeutic effects of mepirodipine. Cardiovasc Drug Rev 1991;9:340–356.
Kashiwabara T, Nakayama K, Yamada S, Tanaka Y. Long-lasting and potent Ca antagonistic actions of a novel dihydropyridine with two asymmetric centers (YM-09730-5) assessed in the porcine coronary artery. Jpn J Pharmacol 1987;(Suppl. 43):731.
Nakayama K, Kashiwabara S, Yamada S, et al. Assessment in pig coronary artery of long-lasting and potent calcium antagonist actions of the novel dihydropyridine derivative mepirodipine hydrochloride. Arzneimittelforschung 1989;39:50–55.
Bauer P. Multiple testing in clinical trials. Stat Med 1991;10:871–890.
D'Hont G, Meurant JP, Clement DL, et al. Long-term (2-year) isradipine data in the treatment of mild-to-moderate hypertension. J Cardiovasc Pharmacol 1992;19(Suppl. 3):S46–S48.
Nami R, Rizzini P, Buracchi P, Pavese G, Gennari C. Long-term antihypertensive treatment with lacidipine, a new long-acting calcium antagonist. J Cardiovasc Pharmacol 1991;18(Suppl. 11):S22–S25.
Tourkantonis A, Lasaridis A, Settas L. Clinical experience with long-term nitrendipine treatment in essential hypertension. J Cardiovasc Pharmacol 1984;6:S1090–S1095.
Mehta JL, Lopez LM, Vlachakis ND, et al. Double-blind evaluation of the dose-response relationship of amlodipine in essential hypertension. Am Heart J 1993;125:1704–1710.
Saragoça AM, Portela JE, Abreu P, et al. Regression of left ventricular hypertrophy in the short-term treatment of hypertension with isradipine. Am J Hypertens 1991;4:S188–S190.
Cruickshank JM, McAinsh J. Patient compliance on taking cardiovascular drug therapy. Acta Therapy 1992;18:53–60.
Omvik P, Thaulow E, Herland OB, Eide I, Midha R, Turner RR. A double-blind, long-term, comparative study on quality of life, safety, and efficacy during treatment with amlodipine or enalapril in mild or moderate hypertensive patients: A multicenter study. J Cardiovasc Pharmacol 1993;22(Suppl. A):S13–S19.
Frick MH, McGibney D, Tyler HM. A dose-response study of amlodipine in mild to moderate hypertension. J Intern Med 1989;225:101–105.
Ventura HO, Messerli FH, Oigman W, Dunn FG, Reisin E, Frohlich ED. Immediate hemodynamic effects of a new calcium channel blocking agent (nitrendipine) in essential hypertension. Am J Cardiol 1983;51:783–786.
Ferrari R, Cucchini F, Bolognesi R, et al. How do calcium antagonists differ in clinical practice? Cardiovasc Drugs Ther 1994;8:565–575.
Dougall HT, McLay J. A comparative review of the adverse effects of calcium antagonists. Drug Safety 1996;15:91–106.
Author information
Authors and Affiliations
Additional information
On behalf of the Dutch Barnidipine Multicenter Study Group
On behalf of the Dutch Barnidipine Multicenter Study Group
Rights and permissions
About this article
Cite this article
Hart, W., Holwerda, N.J. Barnidipine, a Novel Calcium Antagonist for Once-Daily Treatment of Hypertension: A Multicenter, Double-Blind, Placebo-Controlled, Dose-Ranging Study. Cardiovasc Drugs Ther 11, 637–643 (1997). https://doi.org/10.1023/A:1007778706354
Issue Date:
DOI: https://doi.org/10.1023/A:1007778706354