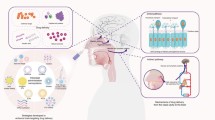
Abstract
Purpose. To study the utility of the nasal route for thesystemic delivery of L-dopa using water soluble prodrugs of L-dopa and toexamine if this delivery method will result in preferential delivery to theCNS.
Methods. Several alkyl ester prodrugs of L-dopa wereprepared and their physicochemical properties were determined. Invitro hydrolysis rate constants in buffer, rat plasma, rat brainhomogenate, rat CSF, and rat nasal berfusate were determined by HPLC. Invivo nasal experiments were carried out in rats. Levels of L-dopa anddopamine in plasma, CSF, and olfactory bulb were determined using HPLCmethod with electrochemical detection.
Results. All the prodrugs showed improved solubility andlipophilicity with relatively fast in vitro conversion in ratplasma. Absorption was fast following nasal delivery of the prodrugs withbioavailability around 90%. Dopamine plasma levels did not changesignificantly following nasal administration of the butyl ester prodrug.Olfactory bulb and CSF L-dopa concentration were higher following nasaldelivery of the butyl ester prodrug compared to an equivalent intravenousdose.
Conclusions. Utilization of water soluble prodrugs ofL-dopa via the nasal route in the treatment of Parkinson's disease may havetherapeutic advantages such as improved bioavailability, decreased sideeffects, and potentially enhanced CNS delivery.
Similar content being viewed by others
REFERENCES
D. Standaert and A. Young. Treatment of Central Nervous System Degenerative Disorders. In A. G. Gilman, J. G. Hardman, L. E. Limbird, P. B. Molinoff, and R. W. Ruddon (eds.), The Pharmacological Basis of Therapeutics, McGraw Hill, New York, 1996 pp. 503–519.
K. Sasahara, T. Nitanai, T. Habara, T. Morioka, and E. Nakajima. Dosage form design for improvement of bioavailability of levodopa V: Absorption and metabolism of levodopa in intestinal segments of dogs. J. Pharm. Sci. 70:1157–1160 (1981).
K. Sasahara, T. Nitanai, T. Habara, T. Morioka, and E. Nakajimai Dosage form design for improvement of bioavailability of levodopa III: Influence of dose on pharmacokinetic behavior of levodopa in dogs and parkinsonian patients. J. Pharm. Sci. 69:1374–1378 (1980).
J. G. Nutt, W. R. Woodward, and J. L. Anderson. The Effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: The mechanism of action in the treatment of parkinsonism. Annal. Neurol. 18:537–543 (1986).
L. B. Wingard, T. M. Brody, J. Larner, and A. Schwartz. Human Pharmacology; Mosby Year Book, St Louis, 1991.
J. M. Cedarbaum. Anti-parkinsonian drugs. Clin. Pharmacokin. 13:141–178 (1987).
J. G. Nutt and W. R. Woodward. Levodopa pharmacokinetics and pharmacodynamics in fluctuating parkinsonian patients. Neurology 36:739–744 (1986).
R. Hardie, A. J. Lees, and G. M. Stern. On-off fluctuations in parkinson's disease. Brain 107:487–506 (1984).
N. Quinn, J. D. Parkes, and C. D. Marsden. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology 34:1131–1136 (1984).
K. Sasahara, T. Nitanai, T. Habara, T. Morioka, Y. Kawahara, and E. Nakajima. Dosage form design for improvement of bioavailability of levodopa IV: Possible causes of low bioavailability of oral levodopa in dogs. J. Pharm. Sci. 70:730–733 (1981).
J. H. Hutton, J. L. Morris, and C. R. Gustavo. Treatment of chronic parkinson's disease with controlled-release carbidopa/levodopa. Neurology 45:861–864 (1988).
N. Bordor, K. B. Sloan, T. Higuchi, and K. Sasahara. Improved delivery through biological membranes 4: Prodrugs of L-dopa. J. Med. Chem. 20:1435–1445 (1977).
D. R. Cooper, C. Marrel, H. van de Waterbeemd, B. Testa, P. Jenner, and C. D. Marsden. L-Dopa esters as potential prodrugs: Behavioural activity in experimental models of parkinson's disease. J. Pharm. Pharmacol. 39:627–635 (1987).
J. A. Fix, J. Alexander, M. Cortese, K. Engle, P. Leppert, and A. J. Repta. Short-chain alkyl esters of L-dopa as prodrugs for rectal absorption. Pharm. Res. 6:501–505 (1989).
A. Hussain, S. Hirai, and R. Bawarshi. Nasal absorption of propranolol from different dosage forms by rats and dogs. J. Pharm. Sci. 69:1411–1413 (1980).
C. H. Huang, R. Kimura, R. B. Nassar, and A. Hussain. Mechanism of nasal absorption of drugs II: Absorption of L-tyrosine and the effect of structural modification on its absorption. J. Pharm. Sci. 74:1298–1301 (1985).
J. A. Faraj, A. A. Hussain, Y. Aramaki, K. Iseki, M. Kagoshima, and L. W. Dittert. Mechanism of nasal absorption of drugs. IV: Plasma levels of radioactivity following intranasal administration of 3H-leucine enkephalin. J. Pharm. Sci. 79:768–770 (1990).
T. Sakane, M. Akizuki, M. Yoshida, S. Yamashita, T. Nadai, M. Hashida, and H. Sezaki. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J. Pharm. Pharmacol. 43:449–451 (1991).
T. Sakane, M. Akizuki, S. Yamashita, T. Nadai, M. Hashida, and H. Sezaki. The transport of drugs to the cerebrospinal fluid directly from the nasal cavity: The relation to the lipophilicity of the drug. Chem. Pharm. Bull. 39:2456–2458 (1991).
T. A. Kumar, G. F. X. David, A. Sankaranarayanan, V. Puri, and K. R. Sundram. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc. Natl. Acad. Sci. 79:4185–4189 (1982).
K. J. Chou and M. D. Donovan. Distribution of antihistamines into the CSF following intranasal delivery. Biopharm. Drug Dispos. 18:335–346 (1997).
A. H. Anton and D. F. Sayre. A Study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the Analysis of catecholamines. J. Pharmacol. Exp. Ther. 138:360–375 (1962).
R. P. Patel and S. Price. Synthesis of benzyl esters of α-amino acids. J. Org. Chem. 30:3573–3577 (1965).
R. C. Causon, M. J. Brown, K. Leenders, and L. Wolfson. HPLC with amperiometric detection of plasma L-3,4-dihydroxyphenylalanine in parkinsonian patients. J. Chromatography 277:115–123 (1983).
A. Hussain. Intranasal Drug Delivery. Adv. Drug Deliv. Rev. 29:39–49 (1998).
C. H. Huang, R. Kimura, R. B. Nassar, and A. Hussain. Mechanism of nasal absorption of drugs. I: Physicochemical parameters influencing the rate of in situ nasal absorption of drugs in rats. J. Pharm. Sci. 74:608–611 (1985).
R. T. Johnson and C. A. Mims. Pathogenesis of viral infectin of the nervous system. New Eng. J. Med. 278:23–30 (1968).
A. Czerniawska. Experimental investigations on the penetration of 198Au from nasal mucosa membrane into cerebrospinal fluid. Acta Oto-Laryngol. 70:58–61 (1970).
J. Liu, W. H. Frey, and Y. E. Rahman. New approach to alzheimer therapy: Delivery of nerve growth factor (NGF) to the brain through the olfactory pathway and assessment of NGF integrity. Pharm. Res. 11:S208 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kao, H.D., Traboulsi, A., Itoh, S. et al. Enhancement of the Systemic and CNS Specific Delivery of L-Dopa by the Nasal Administration of Its Water Soluble Prodrugs. Pharm Res 17, 978–984 (2000). https://doi.org/10.1023/A:1007583422634
Issue Date:
DOI: https://doi.org/10.1023/A:1007583422634