Abstract
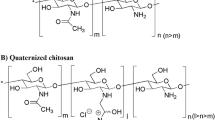
Purpose. To evaluate N-trimethyl chitosan chloride (TMC) of highdegrees of substitution as intestinal permeation enhancers for thepeptide drug buserelin in vitro using Caco-2 cell monolayers, and toinvestigate TMCs as enhancers of the intestinal absorption of buserelinin vivo, in rats.
Methods. TMCs were tested on Caco-2 cells for their efficiency toincrease the paracellular permeability of the peptide buserelin. For thein vivo studies male Wistar rats were used and buserelin wasadministered with or without the polymers intraduodenally. Both types ofexperiments were performed at pH 7.2.
Results. Transport studies with Caco-2 cell monolayers confirmed thatthe increase in buserelin permeation is dependent on the degree oftrimethylation of TMC. In agreement with the in vitro results, in vivodata revealed highly increased bioavailability of buserelin followingintraduodenal co-administration with 1.0% (w/v) TMCs.Intraduodenally applied buserelin resulted in 0.8% absolute bioavailability,whereas co-administrations with TMCs resulted in mean bioavailabilityvalues between 6 and 13 %. Chitosan HCl (1.0% pH = 7.2) did notsignificantly increase the intestinal absorption of buserelin.
Conclusions. Both the in vitro and in vivo results indicate that TMCsare potent mucosal permeation enhancers of the peptide drug buserelinat neutral pH values.
Similar content being viewed by others
REFERENCES
B. J. Aungst, H. Saitoh, D. L. Burcham, S.-M. Huang, S. A. Mousa, and M. A. Hussain. Enhancement of the intestinal absorption of peptides and non-peptides. J. Contr. Rel. 41:19–31 (1996).
G. M. Pauletti, S. Gangwar, T. J. Siahaan, J. Aube, and R. T. Borchardt. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv. Drug Del. Rev. 27:235–256 (1997).
A. Fasano. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 16:152–157 (1998).
P. Artursson, T. Lindmark, S. S. Davis, and L. Illum. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 11:1358–1361 (1994).
L. Illum, N. F. Farraj, and S. S. Davis. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 11:1186–1189 (1994).
H. L. Lueßen, B. J. de Leeuw, M. W. Langemeßer, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm. Res. 13:1668–1672 (1996).
O. Felt, P. Buri, and R. Gurny. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 24:979–993 (1998).
L. Illum. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 15:1326–1331 (1998).
A. F. Kotzé, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. Chitosan for enhanced intestinal permeability: Prospects for derivatives soluble in neutral and basic environments. Eur. J. Pharm. Sci. 7:145–151 (1999).
A. F. Kotzé, M. M. Thanou, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: In vitro evaluation in intestinal epithelial cells (Caco-2). J. Pharm. Sci. 88:253–257 (1999).
M. Thanou, A. F. Kotzé, T. Scharringhausen, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. Effect of degree of quaternization of N-Trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Contr. Rel. (1999). In Press.
M. M. Thanou, J. C. Verhoef, S. G. Romeijn, J. F. Nagelkerke, F. W. H. M. Merkus, and H. E. Junginger. Effects of N-trimethyl chitosan chloride, a novel absorption enhancer, on Caco-2 intestinal epithelia and the ciliary beat frequency of chicken embryo trachea. Int. J. Pharm. 185:73–82 (1999).
A. F. Kotzé, M. Thanou, J. C. Verhoef, and H. E. Junginger. Chitosan and N-trimethyl chitosan chloride as absorption enhancers for nasal and rectal delivery of insulin. Proceed. Intern. Control. Rel. Bioact. Mater. 25:479–480 (1998).
A. B. Sieval, M. Thanou, A. F. Kotzé, J. C. Verhoef, J. Brussee, and H. E. Junginger. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 36:157–165 (1998).
G. Borchard, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, C.-M. Lehr, and H. E. Junginger. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. 3. Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Contr. Rel. 39:131–138 (1996).
A. F. Kotzé, B. J. de Leeuw, H. L. Lueßen, A. G. de Boer, J. C. Verhoef, and H. E. Junginger. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: in vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 159:243–253 (1997).
H. M. Behre, J. Sandow, and E. Nieschlang. Pharmacokinetics of the gonadotropin-releasing hormone agonist buserelin after injection of a slow release preparation in normal men. Arz.-Forsch./Drug Res. 42:80–84 (1992).
M. Gibaldi and D. Perrier. Pharmacokinetics. J. Swarbrick (ed), Drugs and the pharmaceutical sciences. Vol. 1, Marcel Dekker, New York: 409–424 (1975).
A. J. Hoogstraate, J. C. Verhoef, A. Pijpers, L. A. van Leengoed, J. H. M. Verheijden, H. E. Junginger, and H. E. Boddé. In vivo buccal delivery of the peptide drug buserelin with glycodeoxycholate as an absorption enhancer in pigs. Pharm. Res. 13:1233–1237 (1996).
P. Artursson and R. T. Borchardt. Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm. Res. 14:1655–1658 (1997).
F. Delie and W. Rubas. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carr. Syst. 14:221–286 (1997).
H. Lennernäs, K. Palm, U. Fagerholm, and P. Artursson. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 127:103–107 (1996).
T. Lindmark, N. G. Schipper, L. Lazorova, A. G. de Boer, and P. Artursson. Absorption enhancement in intestinal epithelial Caco-2 monolayers by sodium caprate: assessment of molecular weight dependence and demonstration of transport routes. J. Drug Target. 5:215–223 (1998).
P. M. Conn and W. F. Crowley. Gonadotropin-releasing hormone analogues. Annu. Rev. Med. 45:391–405 (1994).
R. Langer. Drug delivery and targeting. Nature 392:5–10 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thanou, M., Florea, B.I., Langemeÿer, M.W.E. et al. N-Trimethylated Chitosan Chloride (TMC) Improves the Intestinal Permeation of the Peptide Drug Buserelin In Vitro (Caco-2 Cells) and In Vivo (Rats). Pharm Res 17, 27–31 (2000). https://doi.org/10.1023/A:1007558206506
Issue Date:
DOI: https://doi.org/10.1023/A:1007558206506