Abstract
Purpose. To determine how the structures of peptides influence theiralveolar permeability.
Methods. The studies were performed using 14 synthetic ‘model’peptides, labelled with a novel, non-intrusive amino acid fluorophore, andtheir transport studied using rat alveolar cell monolayers cultured onpermeable supports.
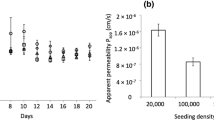
Results. The passage of the peptides across the epithelial cellmonolayers is shown to be primarily paracellular, with an inverse dependenceon molecular size, and an enhanced flux observed for cationic peptides.The apparent permeability coefficients (P app ) for the peptides(together with those for other organic solutes, taken from the literature) areshown to be well-modelled assuming two populations of ‘pores’ in themonolayers, modelled as cylindrical channels of radii 15 Å and 22nm. The former pores are shown to be numerically equatable withthe monolayer tri-junctional complexes, and the latter are taken asmonolayer defects.
Conclusions. The various monolayer P app values correlatewell with the results from in vivo transport experiments, and the conclusion isdrawn that the pulmonary delivery of peptide drugs is perfectlyexploitable.
Similar content being viewed by others
REFERENCES
J. S. Patton, and R. M. Platz. Mechanisms of macromolecule absorption by the lungs, Adv. Drug Del. Rev. 19:3–36 (1996).
D. A. Wall. Pulmonary absorption of peptides and proteins. Drug Delivery 2:1–20 (1995).
L. Wang, D. Toledo-Velasquez, D. Schwegler-Berry, J. K. H. Ma, and Y. Rojanasakul. Transport and hydrolysis of enkephalins in cultured alveolar epithelial cell monolayers. Pharm. Res. 10:1662–1667 (1993).
K. Morimoto, H. Yamahara, V. H. L. Lee, and K.-J. Kim. Dipeptide transport across alveolar epithelial cell monolayers. Pharm. Res. 10:1668–1674 (1993).
H. Yamahara, K. Morimoto, V. H. L. Lee, and K.-J. Kim. Effects of protease inhibitors on vasopressin transport across rat alveolar epithelial cell monolayers. Pharm. Res. 11:1617–1622 (1994).
F. M. Bennett, D. J. Barlow, A. N. O. Dodoo, R. C. Hider, A. B. Lansley, M. J. Lawrence, C. Marriott, and S. S. Bansal. L-(6,7-dimethoxy-4-coumaryl) alanine: an intrinsic probe for the labelling of peptides. Tetrahedron Lett. 38:7449–7452 (1997).
F. M. Bennett, D. J. Barlow, A. N. O. Dodoo, R. C. Hider, A. B. Lansley, M. J. Lawrence, C. Marriott, and S.S. Bansal. Synthesis and properties of (6, 7-dimethoxy-4-coumaryl)alanine:a fluorescent peptide label. Anal. Biochem. 270:15–23 (1999).
K.-J. Kim, D.-K. Suh, R. L. Lubman, S. I., Danto, Z. Borok, and E. D. Crandall. Studies on the mechanisms of action of active ion fluxes across alveolar cell monolayers. J. Tiss. Cult. Meth. 14:187–194 (1992).
C. H. van Os, M. D. de Jong, and J. F. G. Slegers. Dimensions of polar pathways through rabbit gall bladder epithelium, J. Membr. Biol. 15:363–382 (1974).
C. Chothia. Structural invariants in protein folding. Nature 254:304–308 (1975).
R. C. Weast (ed)., CRC Handbook of Chemistry & Physics, CRC Press, Boca Raton Florida, 1987.
B. D. Bunday. Basic Optimisation Methods, Edward Arnold, Victoria, Australia, 1984.
P. Artusson, A.-L. Ungell, and J.-E. Löfroth. Selective permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments. Pharm. Res. 10:1123–1129 (1993).
J. Bertran, A. Werner, G. Stange, D. Markovich, J. Biber, X. Testar, A. Zorzano, M. Palacin, and H. Murer. Expressions of Na+ independent amino acid transport in Xenopus laevis oocytes by injection of rabbit kidney cortex mRNA. Biochem. J. 281:717–723 (1992).
M. Boll, M. Herget, M. Wagener, W. M. Weber, D. Markovich, J. Biber, W. Clauss, H. Murer, and H. Daniel. Expression cloning and functional charaterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc. Natl. Acad. Sci. (USA) 93:284–289 (1996).
Y.-J. Fei, Y. Kanai, S. Nussberger, V. Ganapathy, F. H. Leibach, M. F. Romero, S. K. Singh, W. F. Boron, and M.A. Hediger, Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 368:563–566 (1994).
S. A. Lewis, J. R. Berg, and T. J. Kleine. Modulation of epithelial permeability by extracellular macromolecules. Physiol. Rev. 75:561–589 (1995).
Y. Matsukawa, V. H. L. Lee, E. D. Crandall, and K.-J. Kim. Size-dependent dextran transport across rat alveolar epithelial cell monolayers. J. Pharm. Sci. 86:305–309 (1997).
A. Adson, T. J. Raub, P. S. Burton, C. L. Barsuhn, A. R. Hilgers, K. L. Audus, and N. F. H. Ho. Quantitative approaches to delineate paracellular diffusion in cultured alveolar epithelial cell monolayers. J. Pharm. Sci. 83:1529–1536 (1994).
W. D. Stein. The movement of molecules across cell membranes, Academic Press, New York, 1967.
R. A. Conradi, A. R. Hilgers, N. F. H. Ho, and P. S. Burton. The influence of peptide structure on transport across Caco-2 cells. Pharm. Res. 8:1453–1460 (1991).
T. Teorell. In Butler, J. A. V. and Randall, J. T. (eds). Progress in Biophysics and Biophysical Chemistry, Academic Press, New York, Vol. 3, 1953, pp. 305–369.
F. E. Curry. In Handbook of Physiology. The Cardiovascular System. Microcirculation, Am. Physiol. Soc., Bethesda, MD, sect. 2, vol. IV, pt. 1, 1984, pp. 309–374.
P. Claude. Morphological factors influencing transepithelial permeability; a model for the resistance of the zonula occludens. J. Membr. Biol. 39:219–232 (1978).
J. M. Anderson, M. S. Balda, and A. J. Fanning. The structure and function of tight junctions. Curr. Opin Cell Biol. 5:772–778 (1993).
E. R. Weibel. In Fishman, A.P. and Fisher, A.B. (eds.), Handbook of Physiology. The Respiratory System. Circulation & Non–respiratory functions, Amer. Physiol. Soc., Bethseda, MA, sect. 3, vol. I, 1985, pp. 47–91.
M. M. Berg, K. J. Kim, R. L. Lubman, and E. D. Crandall. Hydrophilic solute transport across rat alveolar epithelium. J. Appl. Physiol. 66:2320–2327 (1989).
L. S. Schanker and J. A. Hemberger. Relation between molecular weight and pulmonary absorption rate of lipid insoluble compounds in neonatal and adult rats. Biochem. Pharmacol. 32:2599–2601 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dodoo, A.N.O., Bansal, S., Barlow, D.J. et al. Systematic Investigations of the Influence of Molecular Structure on the Transport of Peptides Across Cultured Alveolar Cell Monolayers. Pharm Res 17, 7–14 (2000). https://doi.org/10.1023/A:1007514121527
Issue Date:
DOI: https://doi.org/10.1023/A:1007514121527