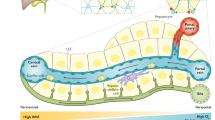
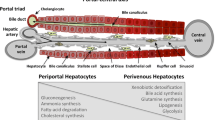
Abstract
The expression of enzymes and other proteins in liver parenchyma is heterogeneous and shows distinct features depending on whether all or only a subset of hepatocytes are involved. Recent advances in the elucidation of the factors that control zonated expression have provided new insights into the regulatory mechanisms that might underlie the different patterns of heterogeneity. While humoral factors seem to play a predominant role in the dynamic adaptation of zonated expression, newly identified intrahepatic (zonation) factors might be responsible for a static dissection of the parenchyma into different expression compartments. These different principles for determining heterogeneous enzyme expression are presented and discussed for cholesterol 7α-hydroxylase and glutamine synthetase.
Similar content being viewed by others
References
Arad G, Freikopf A, Kulka RG. Glutamine stimulated modification and degradation of glutamine synthetase in hepatoma tissue culture cells. Cell. 1976;8:95–101.
Berkowitz CM, Shen CS, Bilir BM, Guibert E, Gumucio JJ.Different hepatocytes express the cholesterol 7 alpha hydroxylase gene during its circadian modulation in vivo. Hepatology. 1995;21:1658–67.
Bralet MP, Branchereau S, Brechot C, Ferry N. Cell lineage study in the liver using retroviral mediated gene transfer. Evidence against the streaming of hepatocytes in normal liver. Am J Pathol. 1994;144:896–905.
Burger H-J, Gebhardt R, Mayer C, Mecke D. Different capacities for amino acid transport in periportal and perivenous hepatocytes isolated by digitonin/collagenase perfusion. Hepatology. 1989;9:22–8.
Clément B, Gugun-Guillouzo C, Campion JP, Glaise D, Bourel M, Guillouzo A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: modulation of albumin secretion and accumulation of extracellular material. Hepatology. 1984;4:373–80.
Corlu A, Kneip B, Lhadi C et al. A plasma membrane protein is involved in cell contact-mediated regulation of tissue-specific genes in adult rat hepatocytes. J Cell Biol. 1991;115:505–15.
Corlu A, Ilyin S, Carion I, Loyer P, Gugun-Guillouzo C. The coculture: a system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol Toxicol. 1997;13:235–42.
Crestani M, Stroup D, Chiang JY. Hormonal regulation of the cholesterol 7 alpha-hydroxylase gene (CYP7). J Lipid Res. 1995;36:2419–32.
Doerner KC, Gurley EC, Vlahcevic ZR, Hylemon PB. Regulation of cholesterol 7 alpha-hydroxylase expression by sterols in primary rat hepatocyte cultures. J Lipid Res. 1995;36: 1168–77.
Fahrner J, Labruyère WT, Gaunitz C, Moorman AFM, Gebhardt R, Lamers WH. Identification and functional characterization of regulatory elements of the glutamine synthetase gene from rat liver. Eur J Biochem. 1993;213: 1067–73.
Gaunitz F, Papke M, Scheja L, Gebhardt R. Role of the glutamine-synthetase promoter for the regulation of the gene. Biol Chem. 1995;376:S99.
Gaunitz F, Gaunitz C, Papke M, Gebhardt R. Cis-regulatory sequences from the first intron of the rat glutamine synthetase gene are involved in hepatocyte specific expression of the enzyme. Biol Chem. 1997;378:11–8.
Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53: 275–354.
Gebhardt R, Mecke D. The role of growth hormone, dexamethasone and triiodothyronine in the regulation of glutamine synthetase in primary cultures of rat hepatocytes. Eur J Biochem. 1979;100:519–25.
Gebhardt R, Mecke D. Heterogenous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2:567–70.
Gebhardt R, Mecke D. Cellular distribution and regulation of glutamine synthetase in liver. In: Häussinger D, Sies H, eds. Glutamine metabolism in mammalian tissues. Berlin: Springer-Verlag; 1984:98–121.
Gebhardt R, Reichen J. Changes in distribution and activity of enzymes associated with ammonia metabolism in CCl4-induced liver cirrhosis in the rat. Hepatology. 1994;20:684–91.
Gebhardt R, Williams GM. Glutamine synthetase and hepatocarcinogenesis. Carcinogenesis. 1995;16:1673–81.
Gebhardt R, Burger H-J, Heini H, Schreiber K-L, Mecke D. Alterations of hepatic enzyme levels and of the acinar distribution of glutamine synthetase in response to experimental liver injury in the rat. Hepatology. 1988a;8:822–30.
Gebhardt R, Ebert A, Bauer G. Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett. 1988b;241:89–93.
Gebhardt R, Lindros K, Lamers WH, Moorman AFM. Hepatocellular heterogeneity in ammonia-metabolism: demonstration of limited colocalization of carbamoyl-phosphate synthetase and glutamine synthetase. Eur J Cell Biol. 1991;56:464–7.
Gebhardt R, Gaunitz F, Mecke D. Heterogeneous (positional) expression of glutamine synthetase: features, regulation and implications for hepatocarcinogenesis. Adv Enzyme Regul. 1994;34:27–56.
Gebhardt R, Wegner H, Alber J. Perifusion of cocultured hepatocytes: optimization of studies on drug metabolism and cytotoxicity in vitro. Cell Biol Toxicol. 1996;12:57–68.
Gebhardt R, Schrode W, Eisenmann-Tappe I. Cellular characteristics of epithelial cell lines from juvenile rat liver: selective induction of glutamine synthetase by dexamethasone. Cell Biol Toxicol. 1997; [in press].
Guguen-Guillouzo C. Isolation and culture of animal and human hepatocytes. In: Freshney RI, ed. Culture of epithelial cells. Glasgow: Alan R. Liss; 1992;1:197–223.
Haupt W. Inhibierende Wirkung eines konditionierten Mediums aus Hepatocytenprimärkulturen auf die Dexamethason-abhängige Induktion der Glutamin Synthetase in der Zellinie RL-ET-1G. Thesis. Tübingen; 1996.
Haupt W, Gebhardt R. Die Glutamin-Synthetase-Induktion durch Dexamethason in Leberepithelzellen steht unter der Kontrolle eines negativen Faktors aus periportalen Ratten-hepatocyten. Z Gastroenterol. 1995;33:53.
Haupt W, Schrode W, Gebhardt R. Conditioned medium from cultured rat hepatocytes completely blocks induction of glutamine synthetase by dexamethasone in several epithelial cell lines. Cell Biol Toxicol. 1997a. [In press].
Haupt W, Gaunitz F, Gebhardt R. Suppression of glutamine synthetase induction in RL-ET-1G by dexamethasone in the presence of conditioned medium from periportal rat hepatocytes is regulated on the post-transcriptional level. Cell Biol Toxicol. 1997b;13:21.
Jungermann K. Zonation of metabolism and gene expression in liver. Histochem Cell Biol. 1995;103:81–91.
Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–51.
Jungermann K, Kietzmann T. Zonation of parenchymal and non-parenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203.
Jungermann K, Thurman RG. Hepatocyte heterogeneity in the metabolism of carbohydrates. Enzyme. 1992;46:33–58.
Jones MP, Pandak WM, Heuman DM, Chiang JY, Hylemon PB, Vlahcevic ZR. Cholesterol 7 alpha hydroxylase: evidence for transcriptional regulation by cholesterol or metabolic products of cholesterol in the rat. J Lipid Res. 1993;34: 885–92.
Kennan AL. Effect of insulin on glutamine synthetase activity in rat liver. Endocrinology. 1964;74:518–20.
Kennedy S, Rettinger S, Flye MW, Parker Ponder K. Experiments in transgenic mice show that hepatocytes are the source for postnatal growth and do not stream. Hepatology. 1995;22:160–8.
Kietzmann T, Jungermann K. Modulation by oxygen of zonal gene expression in liver studied in primary rat hepatocyte cultures. Cell Biol Toxicol. 1997;13:243–55.
Kren BT, Rodrigues CM, Setchell KD, Steer CJ. Posttranscriptional regulation of mRNA levels in rat liver associated with deoxycholic acid feeding. Am J Pathol. 1995;269:G961–73.
Kulka RG, Cohen H. Regulation of glutamine synthetase activity of hepatoma tissue culture cells by glutamine and dexamethasone. J Biol Chem. 1973;248:6738–43.
Lavery DJ, Schibler U. Circadian transcription of cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993;7:1871–84.
Lie-Venema H, Labruyere WT, van Roon MA et al. The spatiotemporal control of the expression of glutamine synthetase in the liver is mediated by its 5'-enhancer. J Biol Chem. 1995;270:28251–6.
Lindros KO. Zonation of cytochrome P450, drug metabolism and toxicity in liver. Gen Pharmacol. 1997;28:191–6.
Maruyama H, Tanaka T, Gebhardt R, Berghem L, Williams GM. Decreased fibronectin in rat liver altered foci and adenomas induced by N-2-fluorenylacetamide. Lab Invest. 1988;58:630–5.
Martinez-Hernández A, Amenta PS. Morphology, localization, and origin of the hepatic extracellular matrix. In: Zern MA, Reid LM, eds. Extracellular matrix. New York: Marcel Dekker; 1993:255–327.
Michaelson J. Biology of disease. Cellular selection in the genesis of multicellular organization. Lab Invest. 1993;69: 136–51.
Moorman AFM, de Boer PAJ, Geerts WJC, van de Zande L, Lamers WH, Charles R. Complementary distribution of carbamoylphosphate synthetase (ammonia) and glutamine synthetase in rat liver acinus is regulated at a pretranslational level. J Histochem Cytochem. 1988;36:751–5.
Pandak WM, Vlahcevic ZR, Heuman DM, Redford KS, Chiang JY, Hylemon PB. Effects of different bile salts on steady-state mRNA levels and transcriptional activity of cholesterol 7 alpha-hydroxylase. Hepatology. 1994;19:941–7.
Quistorff B, Katz N, Witter LA. Hepatocyte heterogeneity in the metabolism of fatty acids: discrepancies on zonation of acetyl-CoA carboxylase. Enzyme. 1992;46:59–71.
Schöls L, Mecke D, Gebhardt R. Reestablishment of the heterogeneous distribution of hepatic glutamine synthetase during regeneration after CCl4-intoxication. Histochemistry. 1990;94:49–54.
Schörner D, Gebhardt R. Okadainsäure hemmt die Induktion der Glutamin Synthetase in cokultiverten Schweinehepatocyten. Z Gastroenterol. 1996;34:67.
Schrode W, Mecke D, Gebhardt R. Induction of glutamine synthetase in periportal hepatocytes by cocultivation with a liver epithelial cell line. Eur J Cell Biol. 1990;53:35–41.
Schrode W. Regulation der Glutaminsynthetase in periportalen Hepatocyten und epitheloiden Zellinien aus Rattenleber sowie deren Co-Kulturen: Bedeutung von Zell-Zell-Wech-selwirkungen. Thesis. Tübingen; 1992.
Schuler M, Gebhardt R. Spontaneous induction of glutamine synthetase expression in pig hepatocytes cocultured with RL-RT-14 cells is completely inhibited by triiodothyonine. Eur J Cell Biol. 1994;63(supplement 40):66.
Shefer S, Kren BT, Salen G et al. Regulation of bile acid synthesis by deoxycholic acid in the rat: different effects on cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase. Hepatology. 1995;22:1215–21.
Shiojiri N, Wada J, Tanaka T, N oguchi M, Ito M, Gebhardt R. Heterogeneous expression of glutamine synthetase by hepatocytes in development of mouse liver. Lab Invest. 1995;72: 740–7.
Sirma H, Williams GM, Gebhardt R. Strain-and sex-specific variations in hepatic glutamine synthetase activity and distribution in rats and mice. Liver. 1996;16:166–73.
Smith DD, Campbell JW Jr. Distribution of glutamine synthetase and carbamoyl-phosphate synthetase I in vertebrate liver. Proc Natl Acad Sci USA. 1988;85:160–4.
Stravitz RT, Hylemon PB, Heuman DM et al. Transcriptional regulation of cholesterol 7 alpha-hydroxylase mRNA by conjugated bile acids in primary cultures of rat hepatocytes. J Biol Chem. 1993;268:13987–93.
Stravitz RT, Vlahcevic ZR, Gurley EC, Hylemon PB. Repression of cholesterol 7 alpha-hydroxylase transcription by bile acids is mediated through protein kinase C in primary cultures of rat hepatocytes. J Lipid Res. 1995;36:1359–69.
Tokumo K, Umemura T, S irma H, Gebhardt R, Poirier M, Williams GM. Inhibition of gonadectomy of effects of 2-acetylaminofluorene in male, but not female rat liver. Carcinogenesis. 1993;14:1747–50.
Twisk J. Regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in cultured rat hepatocytes. Thesis. Leiden; 1994.
Twisk J, Lehmann EM, Princen HMG. Differential feedback regulation of cholesterol 7α-hydroxylase mRNA and transcriptional activity by rat bile acids in primary monolayer cultures of rat hepatocytes. Biochem J. 1993;290:685–91.
Twisk J, Hoekman MF, Müller LM et al. Structural aspects of bile acids involved in the regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase. Eur J Biochem. 1995a;228:596–604.
Twisk J, Hoekman MF, Lehmann EM, Meijer P, Mager WH, Princen HM. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology. 1995b;21:501–10.
Twisk J, Hoekman MF, Mager WH et al. Heterogeneous distribution of cholesterol 7α-hydroxylase and sterol 27-hydroxylase in the rat liver acinus is regulated at the mRNA and translational level. J Clin Invest. 1995c;95:1235–43.
Ugele B, Kempen HJM, Gebhardt R, Meijer P, Burger HJ, Princen HMG. Heterogeneity of liver parenchyma in cholesterol 7α-hydroxylase and bile acid synthesis. Biochem J. 1991;276:73–7.
Wagenaar GT, Chamuleau RA, de Haan JG et al. Experimental evidence that the physiological position of the liver within the circulation is not a major determinant of zonation of gene expression. Hepatology. 1993;18:1144–53.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gebhardt, R., Gaunitz, F. Cell-cell interactions in the regulation of the expression of hepatic enzymes. Cell Biol Toxicol 13, 263–273 (1997). https://doi.org/10.1023/A:1007431307300
Issue Date:
DOI: https://doi.org/10.1023/A:1007431307300