Abstract
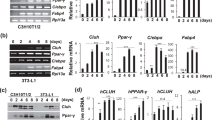
Mitochondrial and cytosolic 3-hydroxy-3-methylglutaryl CoA synthase (m-HMS and c-HMS) genes show high identity at the nucleotide and amino acid level, but no homology has been found in the promoter area. The main regulator for c-HMS is SREBP. The best known transcription factor that regulates m-HMS is PPARα. Three types of PPAR, α, γ and δ have been described in vertebrates. Here we found that they display distinct ligand response profiles in the m-HMS promoter. In some conditions PPARγ is a significant activator of m-HMS. Thus, the m-HMS gene is transiently expressed during the clonal expansion phase of 3T3-L1 differentiation. We found that C/EBPδ and PPARγ activate the m-HMS promoter in 3T3-L1 cells synergistically. This synergistic effect was only observed in the whole promoter (–1148 to +28). A small construct (–116 to +28) which contains the PPRE did not respond to C/EBPδ and/or PPARγ. This suggests that a putative C/EBP site lie somewhere between –1148 and –116 bp. We also show that C/EBPδ was more efficient that C/EBPα and C/EBPβ to activate the m-HMS promoter. The time course of c-HMS mRNA expression during 3T3-L1 differentiation was different, with a significant increase at terminal adipogenesis. We found that the transcription factor C/EBPα did not activate the c-HMS promoter. The differential pattern of expression shown by these two genes, which have a common ancestor, exemplifies refinements of transcriptional control during evolution.
Similar content being viewed by others
References
Ayte J, Gil-Gomez G, Haro D, Marrero PF, Hegardt FG: Rat mitochondrial and cytosolic 3-hydroxy-3methylglutaryl-CoA synthases are encoded by two different genes. Proc Natl Acad Sci USA 87: 3874–3878, 1990
Boukaftabe Y, Duncan A, Wang S, Labuda D, Robert M-F, Sarrazin J, Schappert K, Mitchell GA: Human mitochondrial HMG CoA synthase: Liver cDNA and partial genomic cloning, chromosome mapping to 1p12-p13, and possible role in vertebrate evolution. Genomics 23: 552–559, 1994
Metherall JE, Goldstein JL, Luskey KL, Brown MS: Loss of transcriptional repression of three sterol-regulated genes in mutant hamster cells. J Biol Chem: 264: 15634–15641, 1989
Hegardt FG: Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: A control enzyme in ketogenesis. Biochem J 338: 569–582, 1999
Royo T, Pedragosa MJ, Ayte J, Gil-Gomez G, Vilaro S, Hegardt FG: Testis and ovary express the gene for the ketogenic mitochondrial 3-hydroxy-3methylglutaryl-CoA synthase. J Lipid Res 34: 867–874, 1993
Serra D, Asins G, Hegardt FG: Ketogenic mitochondrial 3-hydroxy 3-methylglutaryl-CoA synthase gene expression in intestine and liver of suckling rats. Arch Biochem Biophys 301: 445–448, 1993
Clinkerbeard KD, Sugiyama T, Reed WD, Lane MD: Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from liver. J Biol Chem 250: 3124–3135, 1975
Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS: Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 99: 838–845, 1997
Tontonoz P, Kim JB, Graves RA, Spiegelman BM: ADD1: A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol 13: 4753–4759, 1993
Miserez AR, Cao G, Probst L, Hobbs HH: Structure of the human gene encoding sterol regulatory element binding protein 2 (SREBF2). Genomics 40: 31–40, 1997
Brown MS, Goldstein JL: The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89: 331–340, 1997
Brown MS, Goldstein JL: A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA 96: 11041–11048, 1999
Dooley KA, Millinder S, Osborne TF: Sterol regulation of 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene through a direct interaction between sterol regulatory element binding protein and the trimeric CCAAT-binding factor/nuclear factor Y. J Biol Chem 16: 1349–1356, 1998
Dooley KA, Bennett MK, Osborne TF: A critical role for cAMP response element-binding protein (CREB) as a Co-activator in sterolregulated transcription of 3-hydroxy-3-methylglutaryl coenzyme A synthase promoter. J Biol Chem 274: 5285–5291, 1999
Bennett MK, Lopez JM, Sanchez HB, Osborne TF: Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem 270: 25578–2558, 1995
Lopez JM, Bennett MK, Sanchez HB, Rosenfeld JM, Osborne TF: Sterol regulation of acetyl CoA carboxylase: A mechanism for coordinate control of cellular lipid. Proc Natl Acad Sci USA 93: 1049–1053, 1996
Kim JB, Spiegelman BM: ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 10: 1096–1107, 1996
Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL: Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest 98: 1575–1584, 1996
Rodriguez JC, Gil-Gomez G, Hegardt FG, Haro D: Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J Biol Chem 269: 18767–18772, 1994
Eggers A, Caudevilla C, Asins G, Hegardt FG, Serra D: Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase promoter contains a CREB binding site that regulates cAMP action in Caco-2 cells. Biochem J 345: 201–206, 2000
Schoonjans K, Staels B, Auwerx J: Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37: 907–925, 1996
Zhu Y, Qi C, Korenberg JR, Chen X-N, Noya D, Rao MS, Reddy JK: Structural organization of mouse peroxisome proliferator activated receptor γ (mPPARγ) gene: Alternative promoter use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci USA 92: 7921–7925, 1995
Tontonoz P, Hu E, Spiegelman BM: Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79: 1147–1156, 1994
Spiegelman BM, Flier JS: Adipogenesis and obesity: Rounding out the big picture. Cell 87:377–389, 1996
Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J: Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol Cell Biol 19: 5495–5503, 1999
Kim JB, Wright HM, Wright M, Spiegelman BM: ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc Natl Acad Sci USA 95: 4333–4337, 1998
Cornelius P, MacDougald OA, Lane MD: Regulation of adipocyte development. Annu Rev Nutr 14: 99–129, 1994
Yeh W-C, Li T-K, Bierer BE, McKnight SL: Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proc Natl Acad Sci USA 92: 11081–11085, 1995
Cao Z, Umek R, McKnight SL: Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5: 1538–1552, 1991
Tanaka T, Yoshida N, Kishimoto T, Akira S: Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J 16: 7432–7443, 1997
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K: In: Current Protocols in Molecular Biology. Green Publishing Associates/Wiley-Interscience, New York, 1987, pp 9.1.4–9.1.6
Sambrook J, Fritsch EF, Maniatis T: In: Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1989
Fineberg AP, Vogelstein B: A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem 137: 266–267, 1984
Gil-Gómez G, Ayte J, Hegardt FG: The rat mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A-synthase gene contains elements that mediate its multihormonal regulation and tissue specificity. Eur J Biochem 213: 773–779, 1993
Green S, Issemann I, Sheer E: A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res 16: 369, 1988
Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM: Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA 91: 7355–7359, 1994
Foxworthy PS, Eacho PI: Effect of the peroxisome proliferator LY-171883 on triglyceride accumulation in rats fed a fat-free diet. Biochem Pharmacol 42: 1487–1491, 1991
Yubero P, Manchado C, Cassard-Doulcies A-M, Mampel T, Viñas O, Iglesias R, Giralt M, Villarroya F: CCAAT/Enhancer binding proteins α and β are transcriptional activators of the brown fat uncoupling protein gene promoter. Biochem Biophys Res Commun 198: 653–659, 1994
Zechner R, Moser R, Newman TC, Fried SK, Breslow JL: Apolipoprotein E gene expression in mouse 3T3-L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem 266: 10583–10588, 1991
Nishio E, Tomiyama K, Nakata H, Watanabe Y: 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitor impairs cell differentiation in cultured adipogenic cells (3T3-L1). Eur J Pharmacol 301: 203–206, 1996
Ortiz JA, Gil-Gómez G, Casaroli-Marano RP, Vilaró S, Hegardt FG, Haro D: Transfection of the ketogenic mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase cDNA into Mev-1 cells corrects their auxotrophy for mevalonate. J Biol Chem 269: 28523–28526, 1994
Meertens LM, Miyota KS, Cechetto JD, Rachubinski RA, Capone JP: A mitochondrial ketogenic enzyme regulates its gene expression by association with the nuclear hormone receptor PPARα. EMBO J 17: 6972–6978, 1998
Mascaro C, Acosta E, Ortiz JA, Marrero PF, Hegardt FG, Haro D: Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J Biol Chem 273: 8560–8563, 1998
Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS: Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 99: 838–845, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lopez, J., Hegardt, F. & Haro, D. Differential expression of cytosolic and mitochondrial 3-hydroxy-3-methylglutaryl CoA synthases during adipocyte differentiation. Mol Cell Biochem 217, 57–66 (2001). https://doi.org/10.1023/A:1007284217886
Issue Date:
DOI: https://doi.org/10.1023/A:1007284217886