Abstract
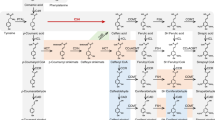
Lignins are phenolic biopolymers synthesized by terrestrial, vascular plants for mechanical support and in response to pathogen attack. Peroxidases have been proposed to catalyse the dehydrogenative polymerization of monolignols into lignins, although no specific isoenzyme has been shown to be involved in lignin biosynthesis. Recently we isolated an extracellular anionic peroxidase, ATP A2, from rapidly lignifying Arabidopsis cell suspension culture and cloned its cDNA. Here we show that the Atp A2 promoter directs GUS reporter gene expression in lignified tissues of transgenic plants. Moreover, an Arabidopsis mutant with increased lignin levels compared to wild type shows increased levels of ATP A2 mRNA and of a mRNA encoding an enzyme upstream in the lignin biosynthetic pathway. The substrate specificity of ATP A2 was analysed by X-ray crystallography and docking of lignin precursors. The structure of ATP A2 was solved to 1.45 Å resolution at 100 K. Docking of p-coumaryl, coniferyl and sinapyl alcohol in the substrate binding site of ATP A2 were analysed on the basis of the crystal structure of a horseradish peroxidase C-CN-ferulic acid complex. The analysis indicates that the precursors p-coumaryl and coniferyl alcohols are preferred by ATP A2, while the oxidation of sinapyl alcohol will be sterically hindered in ATP A2 as well as in all other plant peroxidases due to an overlap with the conserved Pro-139. We suggest ATP A2 is involved in a complex regulation of the covalent cross-linking in the plant cell wall.
Similar content being viewed by others
References
Barkholt, V. and Jensen, A.L. 1989. Amino acid analysis: dertermination of cysteine plus half-cystine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal. Biochem. 177: 318–322.
Bechtold, N., Ellis, J. and Pelletier, G.X. 1993. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis plants. C. R. Acad. Sci. Paris. Life Sci. 316: 1194–1199.
Bernards, M.A., Fleming, W.D., Llewellyn, D.B., Priefer, R., Yang, X., Sabatino, A. and Plourde, G.L. 1999. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol. 121: 135–146.
Boudet, A.M., Lapierre, C. and Grima-Pettenati, J. 1995. Biochemistry and molecular biology of lignification. New Phytol. 129: 203–236.
Chabanet, A., Catesson, A.M. and Goldberg, R. 1993. Peroxidase and phenolase activities in mung bean hypocotyl cell walls. Phytochemistry 33: 759–763.
Chabanet, A., Goldberg, R., Catesson, A.M., Quinet-Szely, M., Delaunay, A.M. and Faye, L. 1994. Characterization and localization of a phenol oxidase in mung bean hypocotyl cell walls. Plant Physiol. 106: 1095–1102.
Church, D.L. and Galston, A.W. 1988. 4-Coumarate:coenzyme A ligase and isoperoxidase expression in Zinnia mesophyl cells induced to differentiate into tracheary elements. Plant Physiol. 88: 679–684.
Dunford, H.B. 1991. Horseradish peroxidase: structure and kinetic properties. In: J. Everse and M.B. Grisham (Eds.) Peroxidases in Chemistry and Biology, CRC Press, Boca Raton, FL, pp. 1–24.
Edwards, S.L. and Poulos, T.L. 1990. Ligand binding and structural perturbations in cytochrome c peroxidase: a crystallographic study. J. Biol. Chem. 265: 2588–2595.
Engh, R.A. and Huber, R. 1991. Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr. A47: 392–400.
Finzel, B.C., Poulos, T.L. and Kraut, J. 1984. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-Å resolution. J. Biol. Chem. 259: 13027–13036.
Fukuyama, K., Kunishima, N., Amada, F., Kubota, T. and Matsubara, H. 1995. Crystal structures of cyanide-and triiodide-bound forms of Arthromyces ramosus peroxidase at different pH values. Perturbations of active site residues and their implication in enzyme catalysis. J. Biol. Chem. 270: 21884–21892.
Gajhede, M., Schuller, D.J., Henriksen, A., Smith, A.T. and Poulos, T.L. 1997. Crystal structure of horseradish peroxidase C at 2.15 Å resolution. Nature Struct. Biol. 4: 1032–1038.
Gang, D.R., Costa, M.A., Fujita, M., Dinkova-Kostova, A.T., Wang, H.-B., Burlat, V., Martin, W., Sarkanen, S., Davin, L.B. and Lewis, N.G. 1999. Regiochemical control of monolignol radical coupling: a new paradigm for lignin and lignan biosynthesis. Chem. Biol. 6: 143–151.
Gazarian, I.G., Ashby, G.A., Thorneley, R.N.F. and Lagrimini, L.M. 1996. Study of indole-3-acetic acid oxidation by molecular oxygen catalyzed by horseradish and tobacco peroxidases. In: C. Obinger, U. Burner, R. Ebermann, C. Penel, and H. Greppin (Eds.) Plant Peroxidases: Biochemistry and Physiology, University of Geneva, Geneva, pp. 70–75.
Glusker, J.P. 1991. Structural aspects of ligand binding to functional groups in proteins. Adv. Protein Chem. 42: 1–76.
Henriksen, A., Schuller, D.J., Meno, K., Welinder, K.G., Smith, A.T. and Gajhede, M. 1998. Structural interactions between horseradish peroxidase C and the substrate benzhydroxamic acid determined by X-ray crystallography. Biochemistry 37: 8054–8060.
Henriksen, A., Smith, A.T. and Gajhede, M. 1999. The structures of the horseradish peroxidase C-ferulic acid complex and the ternary complex with cyanide suggest how peroxidases oxidize small phenolic substrates. J. Biol. Chem. 274: 35005–35011.
Higo, K., Ugawa, Y., Iwamoto, M. and Korenaga, T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database. Nucl. Acids Res. 27: 297–300.
Ito, H., Hiraga, S., Tsugawa, H., Matsui, H., Honma, M., Otsuki, Y., Murakami, T. and Ohashi, Y. 2000. Xylem-specific expression of wound-inducible rice peroxidase genes in transgenic plants. Plant Sci. 155: 85–100.
Itzhaki, H., Maxson, J.M. and Woodson, W.R. 1994. An ethyleneresponsive enhancer element is involved in the senescencerelated expression of the carnation glutathione-S-transferase (GST1) gene. Proc. Natl. Acad. Sci. USA 91: 8925–8929.
Jabs, T., Dietrich, R.A. and Dangl, J.L. 1996. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273: 1853–1856.
Jones, A., Zou, J.Y., Cowan, S.W. and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these maps. Acta Crystallogr. A47: 110–119.
Kraulis, P.J. 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24: 946–950.
Lacombe, E., Hawkins, S., Doorsselaere, J.V., Piquemal, J., Goffner, D., Poeydomenge, O., Boudet, A.M. and Grima-Pettenati, J. 1997. Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: cloning, expression and phylogenetic relationships. Plant J. 11: 429–441.
Lagrimini, L.M. 1996. The role of the tobacco anionic peroxidase in growth and development. In: C. Obinger, U. Burner, R. Ebermann, C. Penel, and H. Greppin (Eds.) Plant Peroxidases: Biochemistry and Physiology, University of Geneva, Geneva, pp. 235–242.
Lagrimini, L.M., Burkhart, W., Moyer, M. and Rothstein, S. 1987. Molecular cloning of a complementary DNA encoding the lignin-forming peroxidase from tobacco: molecular analysis and tissue-specific expression. Proc. Natl. Acad. Sci. USA. 84: 7542–7546.
Lagrimini, L.M., Joly, R.J., Dunlap, J.R. and Liu, T.-T.Y. 1997. The consequence of peroxidase overexpression in transgenic plants on growth and development. Plant Mol. Biol. 33: 887–895.
Lee, D., Ellard, M., Wanner, L.A., Davis, K.R. and Douglas, C.J. 1995. The Arabidopsis thaliana 4-coumarate:CoA ligase (4CL) gene: stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol. Biol. 28: 871–884.
Lewis, N.G., Davin, L.B. and Sarkanen, S. 1999. The nature and function of lignins. In: P.M. Pinto (Ed.) Carbohydrates and their Derivatives Including Tannins, Cellulose and Related Lignins, Elsevier, Amsterdam, pp. 617–745.
Lewis, N.G. and Yamamoto, E. 1990. Lignin: occurrence, biogenesis and biodegradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41: 455–496.
Li, L., Popko, J.L., Zhang, X.-H., Osakabe, K., Tsai, C.-J., Joshi, C.P. and Chiang, V.L. 1997. A novel multifunctional O-methyltransferase implicated in a dual methylation pathway associated with lignin biosynthesis in loblolly pine. Proc. Natl. Acad. Sci. USA 94: 5461–5466.
Merritt, E.A. and Bacon, D.J. 1997. Raster3D: photorealistic molecular graphics. Meth. Enzymol. 277: 505–524.
Milanesi, L., Muselli and Arrigo, P. 1996. Hamming clustering method for signals prediction in 5' and 3' regions of eukaryotic genes. Comput. Appl. Biosci. 12: 399–404.
Millar, A.J., Short, S.R., Chua, N.-H. and Kay, S.A. 1992. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4: 1075–1087.
Mohan, R., Bajar, A.M. and Kolattukudy, P.E. 1993. Induction of a tomato anionic peroxidase gene (tap1) by wounding in transgenic tobacco and activation of tap1/GUS and tap2/GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Mol. Biol. 21: 341–354.
Montgomery, J., Goldman, S., Deikman, J., Margossian, L. and Fisher, R.L. 1993. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc. Natl. Acad. Sci. USA 90: 5939–5943.
Mundy, J., Mayer, R. and Chua, N.-H. 1995. Cloning genomic sequences using long-range PCR. Plant Mol. Biol. 13: 156–163.
Mäder, M. and Füssl, R. 1982. Role of peroxidase in lignification of tobacco cells. Plant Physiol. 70: 1132–1134.
Nagy, F., Kay, S.A. and Chua, N.-H. 1988. Analysis of gene expression in transgenic plants. In: S.B. Gelvin and R.A. Schilperoort (Eds.) Plant Molecular Biology Manual, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 1–29.
Nakamura, W. 1967. Studies of the biosynthesis of lignins. I. Disproof against the catalytic activity of laccase in the oxidation of coniferyl alcohol. J. Biochem. 62: 54–61.
Navaza, J. 1994. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A50: 157–163.
Nose, M., Bernards, M.A., Furlan, M., Zajicek, J., Eberhardt, T.L. and Lewis, N.G. 1995. Towards the specification of consecutive steps in macromolecular lignin assembly. Phytochemistry 39: 71–79.
Ogawa, K., Kanematsu, S. and Asada, K. 1996. Intra-and extracellular localization of 'cytosolic' CuZn-superoxide dismutase in spinach leaf and hypocotyl. Plant Cell Physiol. 37: 790–799.
Ogawa, K., Kanematsu, S. and Asada, K. 1997. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vacular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 38: 1118–1126.
Olson, P.D. and Varner, J.E. 1993. Hydrogen peroxide and lignification. Plant J. 4: 887–892.
Østergaard, L., Abelskov, A.K., Mattsson, O. and Welinder, K.G. 1996. Structure and organ specificity of an anionic peroxidase from Arabidopsis thaliana cell suspension culture. FEBS Lett. 398: 243–247.
Otwinowski, Z. and Minor, W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Meth. Enzymol 276: 307–326.
Piquemal, J., Lapierre, C., Myton, K., O'Connell, A., Schuch, W., Grima-Pettenati, J. and Boudet, A.M. 1998. Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J. 13: 71–83.
Poulos, T.L., Edwards, S.L., Wariishi, H. and Gold, M.H. 1993. Crystallographic refinement of lignin peroxidase at 2 Å. J. Biol. Chem. 268: 4429–4440.
Quiroga, M., Guerrero, C., Botella, M.A., Barceló, A., Amaya, I., Medina, M.I., Alonso, F.J., de Forchetti, S.M., Tigier, H. and Valpuesta, V. 2000. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 122: 1119–1127.
Ruch, F. and Bosshardt, U. 1963. Photometrische Bestimmung von Stoffmengen im Fluoreszenz Mikroskop. Z. Wiss. Mikrosk. Mikrosk. Techn. 65: 335–341.
Sato, Y., Sugiyama, M., Komamine, A. and Fukuda, H. 1995. Separation and characterization of the isozymes of wall-bound peroxidase from cultured Zinnia cells during tracheary element differentiation. Planta 196: 141–147.
Shannon, L.M., Kay, E. and Lew, J.Y. 1966. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J. Biol. Chem. 241: 2166–2172.
Sheldrick, G.M. and Schneider, T.R. 1997. SHELXL: highresolution refinement. Meth. Enzymol. 277B: 319–343.
Smith, C.G., Rodgers, M.W., Zimmerlin, A., Ferdinando, D. and Bolwell, G.P. 1994. Tissue and subcellular immunolocalisation of enzymes of lignin synthesis in differentiating and wounded hypocotyl tissue of French bean (Phaseolus vulgaris L.). Planta 192: 155–164.
Smulevich, G., Paoli, M., Burke, J.F., Sanders, S.A., Thorneley, R.-N.F. and Smith, A.T. 1994. Characterization of recombinant horseradish peroxidase C and three site-directed mutants F41V, F41W, and R38K, by resonance Raman spectroscopy. Biochemistry 33: 7398–7407.
Sterjiades, R., Dean, J.F., Gamble, G., Himmelsbach, D.S. and Eriksson, K.-E.L. 1999. Extracellular laccases and peroxidases from sycamore maple (Acer pseudoplatanus) cell suspension cultures. Planta 190: 75–87.
Sundaramoorthy, M., Kishi, K., Gold, M.H. and Poulos, T.L. 1994. The crystal structure of manganese peroxidase from Phanerochaete chrysosporium at 2.06-Å resolution. J. Biol. Chem. 269: 32759–32767.
Sundaresan, V., Springer, P., Volpe, T., Jones, J.D.G., Dean, C., Ma, H. and Martienssen, R. 1995. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9: 1797–1810.
Takahama, U. 1995. Oxidation of hydroxycinnamic acid and hydroxycinnamyl alcohol derivatives by laccase and peroxidase. Interactions among p-hydroxyphenyl, guaiacyl and syringyl groups during the oxidation reaction. Physiol. Plant. 93: 61–68.
Takahama, U. and Oniki, T. 1994. Effects of ascorbate on oxidation of hydroxycinnamic acid derivatives and the mechanism of oxidation of sinapic acid by cell wall bound peroxidases. Plant Cell Physiol. 35: 593–600.
Teilum, K., Østergaard, L. and Welinder, K.G. 1999. Disulfide bond formation and folding of plant peroxidases expressed as inclusion body protein in Escherichia coli thioredoxin reductase negative strains. Protein Expr. Purif. 15: 77–82.
Whetten, R.W., MacKay, J.J. and Sederoff, R.R. 1998. Recent advances in understanding lignin biosynthesis. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 49: 585–609.
Yamazaki, I., Nakajima, R. 1986. Physico-chemical comparison between horseradish peroxidases A and C. In: H. Greppin, C. Penel and Th. Gaspar (Eds.) Molecular and Physiological Aspects of Plant Peroxidases, University of Geneva, Geneva, pp. 71–84.
Ye, Z.-H., Kneusel, R.E., Matern, U. and Varner, J.E. 1994. An alternative methylation pathway in lignin biosynthesis in Zenia. Plant Cell 6: 1427–1439.
Zhong, R., Morrison, H. III, Negrel, J. and Ye, Z.-H. 1998. Dual methylation pathways in lignin biosynthesis. Plant Cell 10: 2033–2045.
Zimmerlin, A., Wojtaszek, P. and Bolwell, G.P. 1994. Synthesis of dehydrogenation polymers of ferulic acid with high specificity by a purified cell-wall peroxidase from French bean (Phaseolus vulgaris L.). Biochem. J. 299: 747–753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Østergaard, L., Teilum, K., Mirza, O. et al. Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of a plant peroxidase with implications for lignification. Plant Mol Biol 44, 231–243 (2000). https://doi.org/10.1023/A:1006442618860
Issue Date:
DOI: https://doi.org/10.1023/A:1006442618860