Abstract
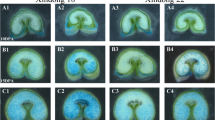
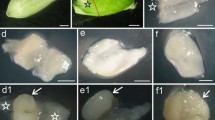
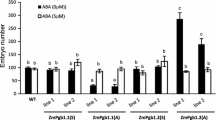
Cereal endosperm undergoes programmed cell death (PCD) during its development, a process that is controlled, in part, by ethylene. Whether other hormones influence endosperm PCD has not been investigated. Abscisic acid (ABA) plays an essential role during late seed development that enables an embryo to survive desiccation. To examine whether ABA is also involved in regulating the onset of PCD during endosperm development, we have used genetic and biochemical means to disrupt ABA biosynthesis or perception during maize kernel development. The onset and progression of cell death, as determined by viability staining and the appearance of internucleosomal DNA fragmentation, was accelerated in developing endosperm of ABA-insensitive vp1 and ABA-deficient vp9 mutants. Ethylene was synthesized in vp1 and vp9 mutant kernels at levels that were 2–4-fold higher than in wild-type kernels. Moreover, the increase and timing of ethylene production correlated with the premature onset and accelerated progression of internucleosomal fragmentation in these mutants. Treatment of developing wild-type endosperm with fluridone, an inhibitor of ABA biosynthesis, recapitulated the increase in ethylene production and accelerated execution of the PCD program that was observed in the ABA mutant kernels. These data suggest that a balance between ABA and ethylene establishes the appropriate onset and progression of programmed cell death during maize endosperm development.
Similar content being viewed by others
References
Abeles, F.B., Morgan, P.W. and Saltveit, M.E. Jr. 1992. Ethylene in Plant Biology, Academic Press, San Diego, CA, 414 pp.
Anderson, M.D., Prasad, T.K., Martin, B.A. and Stewart, C.R. 1994. Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance. Plant Physiol. 105: 331–339.
Aoyagi, S., Sugiyama, M. and Fukuda, H. 1998. BEN1 and ZEN1 cDNAs encoding S1-type DNases that are associated with programmed cell death in plants. FEBS Lett. 429: 134–138.
Arrends, M.J., Morris, R.G. and Wyllie, A.H. 1990. Apoptosis: the role of the endonuclease. Am. J. Path. 136: 593–608.
Bailly, C., Corbineau, F. and Come, D. 1992. The effect of abscisic acid and methyl jasmonate on 1-aminocyclopropane 1-carboxylic acid conversion to ethylene in hypocotyl segments of sunflower seedlings, and their control by calcium and calmodulin. Plant Growth. Reg. 11: 349–355.
Barry, M.A. and Eastman, A. 1992. Endonuclease activation during apoptosis: the role of cytosolic Ca2C and pH. Biochem. Biophys. Res. Com. 186: 782–789.
Bartels, P.G. and Watson, C.W. 1978. Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci. 26: 198–203.
Belefant-Miller, H., Fong, F. and Smith, J.D. 1994. Abscisic acid biosynthesis during corn embryo development. Planta 195: 17–21.
Beers, E.P. and Freeman, T.B. 1997. Proteinase activity during tracheary element differentiation in Zinnia mesophyll cell cultures. Plant Physiol. 113: 873–880.
Bethke, P.C., Lonsdale, J.E., Fath, A. and Jones, R.L. 1999. Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 11: 1033–1045.
Blank, A. and McKeon, T.A. 1989. Single-strand-preferring nuclease activity in wheat leaves is increased in senescence and is negatively photoregulated. Proc. Natl. Acad. Sci. USA 86: 3169–3173.
Bostock, R.M. and Quatrano, R.S. 1992. Regulation of Em gene expression in rice. Interaction between osmotic stress and abscisic acid. Plant Physiol. 98: 1356–1363.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Brown, D.G., Sun, X.M. and Cohen, G.M. 1993. Dexamethasoneinduced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J. Biol. Chem. 268: 3037–3039.
Callard, D., Axelos, M. and Mazzolini, L. 1996. Novel molecular markers for late phases of the growth cycle of Arabidopsis 412 thaliana cell-suspension cultures are expressed during organ senescence. Plant Physiol. 112: 705–715.
Cain, K., Inayat-Hussain, S.H., Kokileva, L. and Cohen, G.M. 1994. DNA cleavage in rat liver nuclei activated by Mg2+ or Ca2+ + Mg2+ is inhibited by a variety of structurally unrelated inhibitors. Biochem. Cell Biol. 72: 631–638.
Chandler, P.M., Zwar, J.A., Jacobsen, J.V., Higgins, T.J.V. and Inglis, A.S. 1984. The effects of gibberellic acid and abscisic acid on α-amylase mRNA levels in barley aleurone layers studies using an α-amylase cDNA clone. Plant Mol. Biol. 3: 407–418.
Chang, S.-C. and Gallie, D.R. 1997. RNase activity decreases following a heat shock in wheat leaves and correlates with its posttranslational modification. Plant Physiol. 113: 1253–1263.
Chen, F. and Foolad, M.R. 1997. Molecular organization of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration. Plant Mol. Biol. 35: 821–831.
Chen, T.C. and Kao, C.H. 1992. Senescence of rice leaves. XXXII. Effects of abscisic acid and benzyladenine on polyamines and ethylene production during senescence. J. Plant Physiol. 139: 617–620.
Collins, R.J., Harmon, B.V., Gobe, G.C. and Kerr, J.F.R. 1992. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int. J. Radiat. Biol. 61: 451–453.
Dusenbury, C.E., Davis, M.A., Lawrence, T.S. and Maybaum, J. 1991. Induction of megabase DNA fragments by 5-fluorodeoxyuridine in human colorectal tumor (HT29) cells. Mol. Pharmacol. 39: 285–289.
Espinas, M.L. and Carballo, M. 1993. Pulsed-field gel electrophoresis analysis of higher-order chromatin structures of Zea mays. Highly methylated DNA in the 50 kb chromatin structure. Plant Mol. Biol. 21: 847–857.
Fong, F., Koehler, D.E. and Smith, J.D. 1983a. Fluridone induction of vivipary during maize seed development. In: J.E. Kruger and D.E. Laberge (Eds.), Third International Symposium on Pre-Harvesting Sprouting in Cereals, Westview Press, Boulder, CO, pp. 188–196.
Fong, F., Smith, J.D. and Koehler, D.E. 1983b. Early events in maize seed development. Plant Physiol. 73: 899–901.
Fuchs, Y. and Lieberman, M. 1968. Effects of kinetin, IAA, and gibberellin on ethylene production, and their interactions in growth of seedlings. Plant Physiol. 42: 2029–2036.
Fukuda, H. 1996. Xylogenesis: initiation, progression, and celldeath. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 299–325.
Fukuda, H. 1997. Tracheary element differentiation. Plant Cell 9: 1147–1156.
Fukuda, H. and Komamin, A. 1983. Changes in the synthesis of RNA and protein during tracheary element differentiation in single cells isolated from the mesophyll of Zinnia elegans. Plant Cell Physiol. 24: 603–614.
Greenberg, J.T. 1996. Programmed cell death: a way of life for plants. Proc. Natl. Acad. Sci. USA 93: 12094–12097.
Groover, A. and Jones, A.M. 1999. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 119: 375–384.
Hattori, T., Vasil, V., Rosenkrans, L., Hannah, L.C., McCarty, D.R. and Vasil, I.K. 1992. The viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Dev. 6: 609–618.
Higgins, T.J.V., Jacobsen, J.V. and Zwar, J.A. 1982. Gibberellic acid and abscisic acid modulate protein synthesis and mRNA levels in barley aleurone layers. Plant Mol. Biol. 1: 191–215.
Hole, D.J., Smith, J.D. and Cobb, B.G. 1989. Regulation of embryo dormancy by manipulation of abscisic acid in kernels and associated cob tissue of Zea mays L. cultured in vitro. Plant Physiol. 91: 101–105.
Johnson-Flanagan, A.M., Huiwen, Z., Thiagarajah, M.R. and Saini, H.S. 1991. Role of abscisic acid in the induction of freezing tolerance in Brassica napus suspension-cultured cells. Plant Physiol. 95: 1044–1048.
Jones, R. and Brenner, M. 1987. Distribution of abscisic acid in maize kernel during grain filling. Plant Physiol. 83: 905–909.
Kondo, K., Watanabe, A. and Imaseki, H. 1975. Relationships in actions of indole acetic acid, benzyladenine and abscisic acid in ethylene production. Plant Cell Physiol. 16: 1001–1007.
Lau, O.L. and Yang, S.F. 1973. Mechanism of a synergistic effect of kinetin on auxin-induced ethylene production: suppression of auxin conjugation. Plant Physiol. 51: 1011–1014.
Le Page-Degivry, M.-T. and Garello, G. 1992. In situ abscisic acid synthesis. A requirement for induction of embryo dormancy in Helianthus annuus. Plant Physiol. 98: 1386–1390.
Le Page-Degivry, M.-T., Barthe, P. and Garello, G. 1990. Involvement of endogenous abscisic acid in onset and release of Helianthus annuus embryo dormancy. Plant Physiol. 92: 1164–1168.
Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R. and Lamb, C. 1996. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6: 427–437.
Lieberman, M. and Kunishi, A.T. 1972. Thought on the role of ethylene in plant growth and development. In: D.J. Carr (Ed.), Plant Growth Substances, Springer-Verlag, Berlin/Heidelberg/New York, pp. 549–560.
Lukaszewski, T.A. and Reid, M.S. 1989. Bulb type flower senescence. Acta Hort. 261: 59–62.
McCabe, P.F., Levine, A., Meijer, P.-J., Tapon, N.A. and Pennell, R.I. 1997. A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 12: 267–280.
McCarty, D.R. 1995. Genetic control and integration of maturation and germination pathways in seed development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 71–93.
McCarty, D.R., Carson, C.B., Stinard, P.S. and Robertson, D.S. 1989. Molecular analysis of viviparous-1: an abscisic acidinsensitive mutant of maize. Plant Cell 1: 523–532.
McCarty, D.R., Hattori, T., Carson, C.B., Vasil, V., Lazar, M. and Vasil, I.K. 1991. The viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 66: 895–905.
Mittler, R. and Lam, E. 1995. Identification, characterization, and purification of a tobacco endonuclease activity induced upon hypersensitive response cell death. Plant Cell 7: 1951–1962.
Mittler, R. and Lam, E. 1997. Characterization of nuclease activities and DNA fragmentation induced upon hypersensitive response cell death and mechanical stress. Plant Mol. Biol. 34: 209–221.
Mittler, R., Shulaev, V. and Lam, E. 1995. Coordinate activation of programmed cell death and defense mechanisms in transgenic tobacco plants expressing a bacterial proton pump. Plant Cell 7: 29–42.
Mittler, R., Simon, L. and Lam, E. 1997. Pathogen-induced programmed cell death in tobacco. J. Cell Sci. 110: 1333–1344.
Morgan, P.W. and Hall, W.C. 1962. Effect of 2,4-dichlorophenoxyacetic acid on the production of ethylene by cotton and grain sorghum. Plant Physiol. 15: 420–427.
Mozer, T.J. 1980. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell 20: 479–485.
Navarre, D.A. and Wolpert, T.J. 1999. Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 11: 237–249.
Neill, S.J., Horgan, R. and Parry, A.D. 1986. The carotenoid and abscisic acid content of viviparous kernels and seedlings of Zea mays L. Planta 169: 87–96.
Ober, E.S. and Sharp, R.E. 1994. Proline accumulation in maize (Zea mays L.) primary roots at low water potentials. I. Requirement for increased levels of abscisic acid. Plant Physiol. 105: 981–987.
Oberhammer, F., Wilson, J.W., Dive, C., Morris, I.D., Hickman, J.A., Wakeling, A.E., Walker, P.R. and Sikorska, M. 1993. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12: 3679–3684.
O'Donnell, P.J., Calvert, C., Atzorn, R., Wasternack, C., Leyser, H.M.O., and Bowles, D.J. 1996. Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917.
Oishi, M.Y. and Bewley, J.D. 1990. Distinction between the responses of developing maize kernels to fluridone and desiccation in relation to germinability, α-amylase activity, and abscisic acid content. Plant Physiol. 94: 592–598.
Oishi, M.Y. and Bewley, J.D. 1992. Premature drying, fluridonetreatment, and embryo isolation during development of maize kernels (Zea mays L.) induce germination, but the protein synthetic responses are different. Potential regulation of germination and protein synthesis by abscisic acid. J. Exp. Bot. 43: 759–767.
Orzaez, D. and Granell, A. 1997. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J. 11: 137–144.
Panavas, T., Walker, E.L. and Rubinstein, B. 1998. Possible involvement of abscisic acid in senescence of daylily petals. J. Exp. Bot. 49: 1987–1997.
Pence, V.C. 1991. Abscisic acid and the maturation of cacao embryos in vitro. Plant Physiol. 98: 1391–1395.
Pennell, R.I. and Lamb, C. 1997. Programmed cell death in plants. Plant Cell 9: 1157–1168.
Rajasekaran, K., Hein, M.B. and Vasil, I.K. 1987. Endogenous abscisic acid and indole-3-acetic acid and somatic embryogenesis in cultured leaf explants of Pennisetum purpureum Schum. Effect in vivo and in vitro of glyphosate, fluridone, and paclobutrazol. Plant Physiol. 84: 47–51.
Rock, C. and Quatrano, R.S. 1994. Insensitivity is in the genes. Curr. Biol. 4: 1013–1015.
Rock, C., and Quatrano, R.S. 1995. The role of hormones during seed development. In: P.J. Davies (Ed.), Plant Hormones: Physiology, Biochemistry and Molecular Biology, 2nd ed., Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 671–697.
Ryerson, D.E. and Heath, M.C. 1996. Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8: 393–402.
Saab, I., Sharp, R.E., Pritchard, J. and Voetberg, G.S. 1990. Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 93: 1329–1336.
Saniewski, M., Nowacki, J. and Czapski, J. 1987a. The effect of methyl jasmonate on ethylene production and ethylene-forming enzyme activity in tomatoes. J. Plant Physiol. 129: 175–180.
Saniewski, M., Urbanek, H. and Czapski, J. 1987b. Effect of methyl jasmonate on ethylene production, chlorophyll degradation, and polygalacturonase activity in tomatoes. J. Plant Physiol. 127: 177–181.
Schwartz, L.M., Smith, S.W., Jones, M.E.E. and Osborne, B.A. 1993. Do all programmed cell deaths occur via apoptosis? Proc. Natl. Acad. Sci. USA 90: 980–984.
Skriver, K. and Mundy, J. 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512.
Solomon, M., Belenghi, B., Delledonne, M., Menachem, E. and Levine, A. 1999. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11: 431–443.
Steinbach, H.S., Benech-Arnold, R.L. and Sanchez, R.A. 1997. Hormonal regulation of dormancy in developing sorghum seeds. Plant Physiol. 113: 149–154.
Thelen, M.P. and Northcote, D.H. 1989. Identification and purification of a nuclease from Zinnia elegans L.: a potential marker for xylogenesis. Planta 179: 181–195.
Tomei, L.D. and Cope, F.O. 1991. Apoptosis: The Molecular Basis of Cell Death, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Tomei, L.D. and Cope, F.O. 1994. Apoptosis II: The Molecular Basis of Apoptosis in Disease, Current Communications in Cell and Molecular Biology, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Van Doorn, W.G. and Stead, A.D. 1994. The physiology of petal senescence which is not initiated by ethylene. In: R.J. Scott and A.D. Stead (Eds.), Molecular and Cellular Aspects of Plant Reproduction, Cambridge University Press, Cambridge, UK, pp. 239–254.
Walker, P.R., Kokileva, L., LeBlanc, J. and Sikorska, M. 1993. Detection of the initial stages of DNA fragmentation in apoptosis. Bio/technology 15: 1032–1040.
Walker, P.R. and Sikorska, M. 1994. Endonuclease activities, chromatin structure, and DNA degradation in apoptosis. Biochem. Cell. Biol. 72: 615–623.
Walker, P.R., Smith, C., Youdale, T., Leblanc, J. Whitfield, J.F. and Sikorska, M. 1991. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 51: 1078–1085.
Wang, M., Hoekstra, S., van Bergen, S., Lamers, G.E.M., Oppedijk, B.J., van der Heijden, M.W., de Priester, W. and Schilperoort, R.A. 1999. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol. Biol. 39: 489–501.
Wang, H., Li, J., Bostock, B.M. and Gilchrist, D.G. 1996a. Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391.
Wang, M., Oppedijk, B.J., Lu, X., van Duijn, B. and Schilperoort, R.A. 1996b. Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol. Biol. 32: 1125–1134.
Woffenden, B.J., Freeman, T.B. and Beers, E.P. 1998. Proteasome inhibitors prevent tracheary element differentiation in Zinnia mesophyll cell cultures. Plant Physiol. 118: 419–430.
Wyllie, A.H. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68: 251–306.
Ye, Z.-H. And Varner, J.E. 1996. Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol. Biol. 30: 1233–1246.
Yeong-Biau, Y. and Yang, S.F. 1979. Auxin-induced ethylene production and its inhibition by aminoethoxyvinylglycine and cobalt ion. Plant Physiol. 64: 1074–1077.
Yoshii, H. and Imaseki, H. 1981. Biosynthesis of auxin-induced ethylene. Effects of indole-3-acetic acid, benzyladenine and abscisic 414 acid on endogenous levels of 1-aminocyclopropane-1-carboxylic acid (ACC) and ACC synthase. Plant Cell Physiol. 22: 369–379.
Young, T.E. and Gallie, D.R. 1999. Analysis of programmed cell death in wheat endosperm reveals differences in endosperm development between cereals. Plant Mol. Biol. 39: 915–926.
Young, T.E., Gallie, D.R. and DeMason, D.A. 1997. Ethylene mediated programmed cell death during maize endosperm development of Su and sh2 genotypes. Plant Physiol. 115: 737–751.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Young, T.E., Gallie, D.R. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol Biol 42, 397–414 (2000). https://doi.org/10.1023/A:1006333103342
Issue Date:
DOI: https://doi.org/10.1023/A:1006333103342