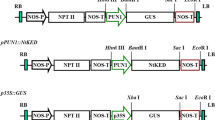
Abstract
The transcription of the tobacco Tnt1 retrotransposon was previously shown to be induced, in tobacco and in heterologous species, by microbial elicitors and by pathogen infections. We report here that the expression of the Tnt1 promoter is also activated in heterologous species such as tomato and Arabidopsis by wounding, freezing and by other abiotic factors known to induce the plant defence response, such as salicylic acid, CuCl2, or oxidative stress. A similar regulation is observed in tobacco for most treatments. The induction of the Tnt1 promoter expression by wounding remains localized around injury points. In CuCl2-treated Arabidopsis plants, the transcription of Tnt1 is correlated with accumulation of the phytoalexin camalexin and with the expression of the EL13 defence gene. The interest of the Tnt1 promoter as a sensitive indicator of the plant defence responses is discussed.
Similar content being viewed by others
References
Arkhipova IR, Ilyin YV: Control of transcription of Drosophila retrotransposons. BioEssays 14: 161–168 (1992).
Bailey JA: Mechanisms of phytoalexin accumulation. In: Bailey JA, Mansfield JW (eds) Phytoalexins, pp. 289–318. Halsted/Wiley, New York (1982).
Boeke JD, Corces VG: Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol 43: 403–434 (1989).
Bowles D: Signals in the wounded plant. Nature 343: 314–315 (1990).
Bowles DJ: Defense-related proteins in higher plants. Annu Rev Biochem 59: 873–907 (1990).
Bradford MM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254 (1976).
Bradshaw VA, McEntee K: DNA damage activates transcription and transposition of yeast Ty retrotransposons. Mol Gen Genet 218: 465–474 (1989).
Casacuberta JM, Grandbastien M-A: Characterisation of LTR sequences involved in the protoplast specific expression of the tobacco Tnt1 retrotransposon. Nucl Acids Res 21: 2087–2093 (1993).
Casacuberta JM, Vernhettes S, Grandbastien M-A: Sequence variability within the tobacco retrotransposon Tnt1 population. EMBO J 14: 2670–2678 (1995).
Chinnadurai G: Modulation of HIV-enhancer activity by heterologous agents: a minireview. Gene 101: 165–170 (1991).
Dangl JL, Lehnacker H, Kiedrowski S, Debener T, Rupprecht C, Arnold M, Somssich I. In: Hennecke H, Verma DPS (eds) Advances in Molecular Genetics of Plant-Microbe Interactions. Current Plant Science and Biotechnology in Agriculture, pp. 78–83. Kluwer Academic Publishers, Dordrecht (1991).
Darwill AG, Albersheim P: Phytoalexins and their elicitors: a defense against microbial infection in plants. Annu Rev Plant Physiol 35: 243–298 (1984).
Dixon RA, Harrison MJ, Lamb CJ: Early events in the activation of plant defense responses. Annu Rev Phytopath 32: 479–501 (1994).
Flavell AJ, Pearce SR, Kumar A: Plant transposable elements and the genome. Curr Opin Gen Devel 4: 838–844 (1994).
Goldsbrough AP, Albrecht H, Stratford R: Salicylic acid-inducible binding of a tobacco nuclear protein to a 10 bp sequence which is highly conserved among stress-inducible genes. Plant J 3: 563–571 (1993).
Grandbastien M-A: Retroelements in higher plants. Trend Genet 8: 103–108 (1992).
Grandbastien M-A, Spielmann A, Caboche M: Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature 337: 376–380 (1989).
Hargreaves JA, Bailey JA: Phytoalexin production by hypocotyls of Phaseolus vulgaris in response to constitutive metabolites releases by damaged bean cells. Physiol Plant Path 13: 89–100 (1978).
Hart CM, Nagy F, Meins FJ: A 61 bp enhancer element of the tobacco β-1,3 glucanase B gene interacts with one or more regulated nuclear proteins. Plant Mol Biol 21: 121–131 (1993).
Hirochika H: Activation of tobacco retrotransposons during tissue culture. EMBO J 12: 2521–2528 (1993).
Hu W, Prem Das O, Messing J: Zeon-1, a member of a new maize retrotransposon family. Mol Gen Genet 248: 471–480 (1995).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Johns MA, Mottinger J, Freeling M: A low copy number, copia-like transposon in maize. EMBO J 4: 1093–1102 (1985).
Junakovic N, Di Franco C, Best-Belpomme M, Echalier G: On the transposition of copia-like nomadic elements in cultured Drosophila cells. Chromosoma 97: 212–218 (1988).
Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S, Ryals J: Induction of systemic acquired disease resistance in plants by chemicals. Annu Rev Phytopath 32: 439–459 (1994).
Kiedrowski S, Kawalleck P, Halbrock K, Somssich IE, Dangl JL: Rapid activation of a novel plant defense gene is strictly dependent on the Arabidopsis RPM1 disease resistance locus. EMBO J 11: 4677–4684 (1992).
Kollmann A, Rouxel T, Bousquet JF: Efficient clean up of non-aqueous plant extracts using reversed-phase cartridges. J Chromatog 473: 293–300 (1989).
Korfhage U, Trezzini GF, Meier I, Hahlbrock K, Somssich IE: Plant homeodomain protein involved in transcriptional regulation of a pathogen defense-related gene. Plant Cell 6: 695–708 (1994).
Loake GJ, Faktor O, Lamb CJ, Dixon RA: Combination of H-box and G-box cis elements is necessary for feed-forward stimulation of a chalcone synthase promoter by the phenylpropanoid-pathway intermediate p-coumaric acid. Proc Natl Acad Sci USA 89: 9230–9234 (1992).
Logemann J, Schell J, Willmitzer J: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Lucas H, Feuerbach F, Kunest K, Grandbastien M-A, Caboche M: The tobacco retrotransposon Tnt1 transposes in Arabidopsis thaliana. EMBO J 14: 2364–2373 (1995).
Malamy J, Klessig DF: Salicylic acid and plant disease resistance. Plant J 2: 643–654 (1992).
Moreau-Mhiri C, Morel J-B, Audéon C, Ferault M, Grandbastien M-A, Lucas H: Regulation of expression of the tobacco Tnt1 retrotransposon in heterologous species following pathogen-related stresses. Plant J 9: 409–419 (1996).
Osterman JC: Transposition ofAc2 in response to temperature. Maydica 36: 147–151 (1991).
Paquin CE, Williamson VM: Effect of temperature on Ty transposition. In: Lambert ME, McDonald JF, Weinstein IB (eds) Eukaryotic Transposable Element as Mutagenic Agents, pp. 235–244. Cold Spring Harbor Press, Cold Spring Harbor, NY (1988).
Pauls PK, Kunert K, Huttner E, Grandbastien MA: Expression of the tobacco Tnt1 retrotransposon promoter in heterologous species. Plant Mol Biol 26: 393–402 (1994).
Pouteau S, Grandbastien M-A, Boccara M: Microbial elicitors of plant defence responses activate transcription of a retrotransposon. Plant J 5: 535–542 (1994).
Pouteau S, Huttner E, Grandbastien M-A, Caboche M: Specific expression of the Tnt1 retrotransposon in protoplasts. EMBO J 10: 1911–1918 (1991).
Rofte M, Spanos A, Banks G: Induction of yeast Ty element transcription by ultraviolet light. Nature 319: 339–340 (1986).
Ryals J, Ukness S, Ward E: Systemic acquired resistance. Plant Plysiol 104: 1109–1112 (1994).
Scandalios JG: Oxygen stress and superoxide dismutases. Plant Physiol 101: 7–12 (1993).
Smyth DR: Dispersed repeats in plant genomes. Chromosoma 100: 355–359 (1991).
Smyth DR: Plant retrotransposons. In: Verma DPS (ed) Control of Plant Gene Expression, pp. 1–15. CRC Press, Boca Raton, FL (1993).
Strand DJ, McDonald JF: Copia is transcriptionally responsive to environmental stress. Nucl Acids Res 13: 4401–4410 (1985).
Suoniemi A, Narvanto A, Schulman AH: The BARE-1 retrotransposon is transcribed in barley from an LTR promoter active in transient assays. Plant Mol Biol 31: 295–306 (1996).
Tsuji J, Jackson EP, Gage DA, Hammerschmidt R, Somerville SC: Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv. syringae. Plant Physiol 98: 1304–1309 (1992).
Varmus H, Brown P: Retroviruses. In: Berg DE, Howe MM (eds) Mobile DNA, pp. 53–108. American Society for Microbiology, Washington, DC (1989).
Vaucheret H, Marion-Poll A, Meyer C, Faure JD, Marin E, Caboche M: Interest in and limits to the utilization of reporter genes for the analysis of transcriptional regulation of nitrate reductase. Mol Gen Genet 235: 259–268 (1992).
Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J: Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance, but is required in signal transduction. Plant Cell 6: 959–965 (1994).
Walbot V: Reactivation of Mutator transposable element of maize by ultraviolet light. Mol Gen Genet 234: 353–360 (1992).
Ziarczyk P, Best-Belpomme M: A short 5′ region of the long terminal repeat is required for regulation by hormone and heat shock of Drosophila retrotransposon 1731. Nucl Acids Res 19: 5689–5693 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mhiri, C., Morel, JB., Vernhettes, S. et al. The promoter of the tobacco Tnt1 retrotransposon is induced by wounding and by abiotic stress. Plant Mol Biol 33, 257–266 (1997). https://doi.org/10.1023/A:1005727132202
Issue Date:
DOI: https://doi.org/10.1023/A:1005727132202