Abstract
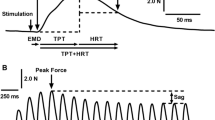
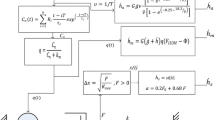
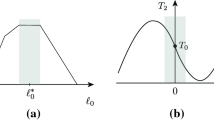
The interactive effects of length and stimulus frequency on rise and fall times and on sag were investigated in fast-twitch feline caudofemoralis at normal body temperature. The length and stimulus frequency ranges studied were 0.8–1.2 L 0 and 15–60 pps. Isometric rise times were shortest under two sets of conditions: short lengths + low stimulus frequencies and long lengths + high stimulus frequencies. In contrast the isometric fall time relationship showed a single minimum at short lengths + low stimulus frequencies. Velocity was shown to have an additional effect on fall time, but only at higher stimulus frequencies (40–60 pps): fall times were shorter during movement in either direction as compared to isometric. The effects of sag were greatest at shorter lengths and lower stimulus frequencies during isometric stimulus trains. Potential mechanisms underlying this last effect were investigated by comparing isometric twitches elicited prior to and immediately following a sag-inducing stimulus train. Post-sag twitches produced less force, reached peak force earlier and initially decayed more quickly compared to pre-sag twitches. However, the final rate of force decay and the initial rate of force rise (during the first 15 ms) were unaffected by sag. We construct a logical argument based on these findings to hypothesize that the predominant mechanism underlying sag is an increase in the rate of sarcoplasmic calcium ion removal. All of the above findings were used to construct a model of activation dynamics for fast-twitch muscle, which was then extrapolated to slow-twitch muscle. When coupled with a previous model of kinematic dynamics, the complete model produced accurate predictions of the forces actually recorded during experiments in which we applied concurrent dynamic changes in length, velocity and stimulus frequency.
Similar content being viewed by others
References
Balnave CD and Allen DG (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol 492: 705–713.
Bellemare F, Woods JJ, Johansson R and Bigland-Ritchie BR (1983) Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50: 1380–1392.
Bigland B and Lippold OCJ (1954) Motor unit activity in the voluntary contraction of human muscle. J Physiol 125: 322–335.
Botterman BR, Iwamoto GA and Gonyea WJ (1986) Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. J Neurophysiol 56: 494–506.
Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269.
Brown IE, Scott SH and Loeb GE (1996) Mechanics of feline soleus: II. Design and validation of a mathematical model. J Muscle Res Cell Motil 17: 219–232.
Brown IE, Satoda T, Richmond FJR and Loeb GE (1998) Feline caudofemoralis muscle. Muscle fiber properties, architecture, and motor innervation. Exp Brain Res 121: 76–91.
Brown IE, Cheng EJ and Loeb GE (1999) Measured and modeled properties of mammalian skeletal muscle: II. The effects of stimulus frequency on force-length and force-velocity relationships. J Muscle Res Cell Motil 20: 627–643.
Brown IE and Loeb GE (1995) A damaged state in cat caudofemoralis muscle induced by high forces. Can J Physiol Pharmacol 73: Ai(Abstract).
Brown IE and Loeb GE (1998a) A physiological model of the dynamic force-producing properties of mammalian fast-twitch skeletal muscle. Soc Neurosci Abstr 24: 1672 (Abstract).
Brown IE and Loeb GE (1998b) Post-activation potentiation — a clue for simplifying models of muscle dynamics. Am Zool 38: 743–754.
Brown IE and Loeb GE (1999) Measured and modeled properties of mammalian skeletal muscle: I. The effects of post-activation potentiation on the time-course and velocity dependencies of force production. J Muscle Res Cell Motil 20: 443–456.
Brown IE and Loeb GE (2000) A reductionist approach to creating and using neuromusculoskeletal models. In: Winters JM and Crago PE (eds.) Biomechanics and Neural Control of Movement, in press, Springer-Verlag New York.
Brown IE and Loeb GE (2000, this issue) Measured and modeled properties of mammalian skeletal muscle: III. The effects of 46 stimulus frequency on stretch-induced force enhancement and shortening-induced force depression.
Burke RE, Levine DN, Tsairis P and Zajac FE (1973) Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748.
Chizeck HJ (1992) Adaptive and nonlinear control methods for neuroprostheses. In: Stein RB, Peckham HP and Popovic D (eds). Neural Prostheses: Replacing motor function after disease or disability, pp. 298–328, Oxford University Press New York.
Close RI (1972) The relations between sarcomere length and charac-teristics of isometric twitch contractions of frog sartorius muscle. J Physiol 220: 745–762.
Cooper S and Eccles JC (1930) The isometric responses of mammalian muscles. J Physiol 69: 377–385.
Durfee WK and Palmer KI (1994) Estimation of force-activation, force-length, and force-velocity properties in isolated, electrically stimulated muscle. IEEE Trans Biomed Eng 41: 205–216.
Gribble PL and Ostry DJ (1996) Origins of the power law relation between movement velocity and curvature: Modeling the effects of muscle mechanics and limb dynamics. J Neurophysiol 76: 2853–2860.
Hatze H (1977) A myocybernetic control model of skeletal muscle. Biol Cybern 25: 103–119.
Hoffer JA, Loeb GE, Sugano N, Marks WB, O'Donovan MJ and Pratt CA (1987a) Cat hindlimb motoneurons during locomotion: III. Functional segregation in sartorius. J Neurophysiol 57: 554–562.
Hoffer JA, Sugano N, Loeb GE, Marks WB, O'Donovan MJ and Pratt CA (1987b) Cat hindlimb motoneurons during locomotion: II. Normal activity patterns. J Neurophysiol 57: 530–553.
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Mol Biol 7: 255–318.
Joyce GS and Rack PMH (1969) Isotonic lengthening and shortening movements of cat soleus muscle. J Physiol 204: 475–491.
Joyce GS, Rack PMH and Westbury DR (1969) Mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol 204: 461–474.
Kernell D, Eerbeek O and Verhey BA (1983) Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction times. Exp Brain Res 50: 220–227.
Krylow AM and Rymer WZ (1997) Role of intrinsic muscle properties in producing smooth movements. IEEE Trans on BME 44: 165–176.
Loeb GE, Brown IE and Cheng EJ (1999) A hierarchical foundation for models of sensorimotor control. Exp Brain Res 126: 1–18.
Otten E (1987) A myocybernetic model of the jaw system of the rat. J Neurosci Meth 21: 287–302.
Powers RKDB and Binder MD (1996) Experimental evaluation of input-output models of motoneuron discharge. J Neurophysiol 75(1): 367–379.
Press WH, Flannery BP, Teukolsky SA and Vetterling WT (1986) Numerical Recipes. Cambridge University Press, Cambridge, USA.
Rack PMH and Westbury DR (1969) The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204: 443–460.
Roszek B, Baan GC and Huijing PA (1994) Decreasing stimulation frequency-dependent length-force characteristics of rat muscle. J Appl Physiol 77: 2115–2124.
Scott SH, Brown IE and Loeb GE (1996) Mechanics of feline soleus: I. Effect of fascicle length and velocity on force output. J Muscle Res Cell Motil 17: 205–218.
Stephenson DG and Wendt IR (1984) Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil 5: 243–272.
Stephenson DG and Williams DA (1982) Effects of sarcomere lengthon the force-pCa relation in fast-and slow-twitch skinned muscle fibres from the rat. J Physiol 333: 637–653.
Westerblad H, Duty S and Allen DG (1993) Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75: 382–388.
Westerblad H and Allen DG (1991) Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98: 615–635.
Zajac FE (1989) Muscle and tendon: Properties, models, scaling and application to biomechanics and motor control. Crit Rev Biomed Engng 17: 359–411.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, I.E., Loeb, G.E. Measured and modeled properties of mammalian skeletal muscle: IV. Dynamics of activation and deactivation. J Muscle Res Cell Motil 21, 33–47 (2000). https://doi.org/10.1023/A:1005687416896
Issue Date:
DOI: https://doi.org/10.1023/A:1005687416896