Abstract
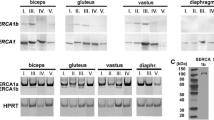
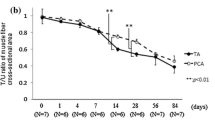
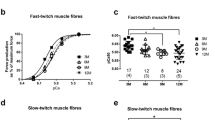
The development of skeletal muscle in mouse oesophagus was investigated by studying the expression of skeletal muscle type myosin heavy chain (MHC), troponin I (TnI) and tropoinin T (TnT) using immunocytochemical and immunoblotting procedures. Both slow and fast muscle fibres were first detected in outer layer muscularis externa of cranial oesophagus at 14 days gestation. The fast MHC was present in all skeletal muscle fibres of oesophagus while the slow MHC was restricted to only a subset of myotubes during foetal development, indicating that slow and fast fibres emerged during early stages of myogenesis. A small number of cells expressed both slow and fast MHCs in the caudal region of adult mouse oesophagus, suggesting that some muscle fibres did not differentiate fully even in the adult. The conversion of some muscle fibre types, from slow to fast, was apparent during postnatal development. This was indicated by a gradual reduction in the number of slow MHC positive fibres during postnatal growth. The complete suppression of slow MHC was observed in cranial oesophagus by 4 weeks of age. However, the persistence of some slow MHC in the caudal oesophagus was apparent even in the adult. The conversion of muscle fibres from slow to fast type was also evidenced by immunoblotting study of fast and slow TnI. The expression level of slow TnI decreased while that of fast TnI increased during neonatal growth period. Compared with the limb skeletal muscles, the onset of the adult fast TnT isoform expression was delayed in mouse oesophagus and its developmental isoforms were not completely suppressed in the adult, although their expression level was reduced.
Similar content being viewed by others
References
Abe H, Komiya T and Obinata T (1986) Expression of multiple troponin T variants in neonatal chicken breast muscle. Dev Biol 118: 42–51.
Bandman E (1992) Contractile protein isoforms in muscle development. Dev Biol 154: 273–383.
Bazhenov DV (1979) Morphohistochemical characteristics of struc-tures of the esophageal cross-striated muscle tissue. Arkh Anat Gistol Embriol 76(1): 26–29.
Briggs MM, Lin JJ and Schachat FH (1987) The extent of amino-terminal heterogeneity in rabbit fast skeletal muscle troponin T. J Muscle Res Cell Motil 8(1): 1–12.
Brooke MH and Kaiser KK (1970) Three human myosin ATPase systems and their importance in muscle pathology. Neurology 20(4): 404–405.
Buckingham ME (1992) Making muscle in mammals. Trends genet 8: 144–149.
Buckingham ME (1994) Muscle: the regulation of myogenesis. Curr Opin Genet Dev 4(5): 745–751.
Buckingham ME (1996) Skeletal muscle development and the role of the myogenic regulatory factors. Biochem Soc Transc 24(2): 506–509.
Buller AJ, Eccles JC and Eccles RM (1960) Interactions between motorneurones and muscle in respect of the characteristic speeds of their contraction. J Physiol 150: 417–439.
Carlson BM (1996) Patten's Foundations of Embryology. 6th edition, McGraw-Hill, New York, p 338.
Crow MT and Stockdale FE (1986) The developmental program of fast myosin heavy chain expression in avian skeletal muscles. Dev Biol 118(2): 333–342.
Dhoot GK (1986) Selective synthesis and degradation of slow skeletal myosin heavy chains in developing muscle fibers. Muscle Nerve 9(2): 155–164.
Dhoot GK(1994) Mammalian myoblasts become fast or slow myocytes within the somitic myotome. J Muscle Res Cell Motil 15(6): 617–622.
Dhoot GK (1988) Identification and distribution of the fast class of troponin T in the adult and developing avian skeletal muscle. J Muscle Res Cell Motil 9(5): 446–455.
Dhoot GK (1990) Isoforms of troponin components in developing muscle fibres. The dynamic state of muscle fibres (ed. D. Pette) Walter de Gruyter, Berlin, New York, 165–179.
Dhoot GK and d'Albis A (1993) Heterogeneity of slow and skeletal myosin heavy chain in foetal rat skeletal muscles. Basic and Appl Myol 3: 181–189.
Dhoot GK and Perry SV (1979) Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature 278(5760): 714–718.
Dubowitz V (1965) Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry 28(6): 516–524.
Ebashi S, Ebashi F and Kodama A (1967) Troponin as the Ca2+-receptive protein in the contractile system. J Biochem 62: 137–138.
Gauthier GF, Lowey S, Benfield PA and Hobbs AW (1982) Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol 92(2): 471–484.
Ghosh S and Dhoot GK (1998a) Both avian and mammalian embryonic myoblasts are intrinsically heterogeneous. J Muscle Res Cell Motil 19(7): 787–795.
Ghosh S and Dhoot GK (1998b) Evidence for distinct fast and slow myogenic cell lineages in human foetal skeletal muscle. J Muscle Res Cell Motil 19(4): 431–441.
Grabarek Z, Drabikowski W, Leavis PC, Rosenfeld SS and Gergely J (1981) Proteolytic fragments of troponin C. Interactions with the other troponin subunits and biological activity. J Biol Chem 256(24): 13121–13127.
Grabarek Z, Grabarek J, Leavis PC and Gergely J (1983) Cooperative binding to the Ca2+-specific sites of troponin C in regulated actin and actomyosin. J Biol Chem 258(23): 14098–14102.
Greaser ML and Gergely J (1971) Reconstitution of troponin activety from three protein components. J Biol Chem 246: 4226–4233.
Gruber H (1968) Structure and innervation of the striated muscle fibres of the esophagus of the rat. Z Zellforsch Mikrosk Anat 91(2): 236–247.
Härtner KT, Kirschbaum BJ and Pette D (1989) The multiplicity of troponin T isoforms. Normal rabbit muscles and effects of chronic stimulation. Eur J Biochem 179: 31–38.
Hoh JF and Hughes S (1991a) Expression of superfast myosin in aneural regenerates of cat jaw muscle. Muscle Nerve 14(4): 316–325.
Hoh JF and Hughes S (1991b) Basal lamina and superfast myosin expression in regenerating cat jaw muscle. Muscle Nerve 14(5): 398–406.
Hughes SM (1993) Muscle development. Running out of control. Nature 364(6437): 485
Jolesz F and Sreter FA (1981) Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol 43: 531–538.
Kugelberg E (1976) Adaptive transformation of rat soleus motor units during growth. J Neurol Sci 27: 269–289.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lowey S, Waller GS and Bandman E (1991) Neonatal and adult myosin heavy chains form homodimers during avian skeletal muscle development. J Cell Biol 113(2): 303–310.
Naumann K and Pette D (1994) Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation 55(3): 203–211.
Nakamura M, Imai H and Hirabayashi T (1989) Coordinate accumulation of troponin subunits in chicken breast muscle. Dev Biol 132: 389–397.
Patapoutian A, Wold BJ and Wagner RA (1995) Evidence for developmentally programmed transdifferentiation in mouse oesophageal muscle. Science 270: 1818–1821.
Perry SV (1979) The regulation of contractile activity in muscle. Biochem Soc Trans 7: 593–617.
Perry SV (1998) Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602.
Peter JB, Barnard RJ, Edgerton VR, Gillespie CA and Stempel KE (1972) Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 11(14): 2627–2633.
Pette D and Vrbova G (1985) Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve 8(8): 676–789.
Pette D and Staron RS (1990) Cellular and molecular diversities of mammalian skeletal muscle fibres. Rev Physiol Biochem Pharmacol 116: 1–76.
Prosser CL (1973) Comparative Animal Physiology, 3rd Edition, Saunders, Philadelphia, pp 719–789.
Sabry MA and Dhoot GK (1989) Identification and distribution of a developmental isoform of troponin I in chicken and rat cardiac muscle. J Muscle Res Cell Motility 10: 85–91.
Sabry MA and Dhoot GK (1991) Identification and pattern of transitions of fast skeletal muscle-like developmental and adult isoforms of troponin T in some rat and human skeletal muscle. J Muscle Res Cell Motility 12: 447–454.
Salmons S and Henriksson J (1981) The adaptive response of skeletal muscle to increased use. Muscle and Nerve 4: 94–102
Schiaffino S and Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76(2): 371–423.
Swynghedsauw B (1986) Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physio Rev 66(3): 710–771.
Towbin H, Staehelin T and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76: 4350–4354.
Weeds AG and Lowey S (1971) Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol 61(3): 701–725.
Whalen RG, Sell SM, Butler-Browne GS, Schwartz K, Bouveret P and Pinset-Harstom I (1981) Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature 292(5826): 805–809.
Whalen RG, Bugaisky LB, Butler-Browne GS and Sell SM (1982) Characterisation of myosin isozymes appearing during rat muscle development. In: Pearson and Epstein (eds.) Muscle Development p. 189–206.
Williams K and Dhoot GK (1992) Heterogeneity and distribution of fast myosin heavy chains in some adult vertebrate skeletal muscles. Histochemistry 97: 479–486.
Zhao W and Dhoot GK (2000a) Both smooth and skeletal muscle precursors are present in foetal mouse oesphagus and they follow different differentiation pathways. Dev Dyn 218: 587–602.
Zhao W and Dhoot GK (2000b) The skeletal muscle precursors in mouse oesophagus are determined during early foetal development. Dev Dyn 219: 10–20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhao, W., Dhoot, G.K. Development and composition of skeletal muscle fibres in mouse oesophagus. J Muscle Res Cell Motil 21, 463–473 (2000). https://doi.org/10.1023/A:1005617419247
Issue Date:
DOI: https://doi.org/10.1023/A:1005617419247