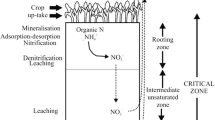
Abstract
A portion of nitrate (NO −3 ), a final breakdown product of nitrogen (N) fertilizers, applied to soils and/or that produced upon decomposition of organic residues in soils may leach into groundwater. Nitrate levels in water excess of 10 mg L−1 (NO3–N) are undesirable as per drinking water quality standards. Nitrate concentrations in surficial groundwater can vary substantially within an area of citrus grove which receives uniform N rate and irrigation management practice. Therefore, differences in localized conditions which can contribute to variations in gaseous loss of NO −3 in the vadose zone and in the surficial aquifer can affect differential concentrations of NO3–N in the groundwater at different points of sampling. The denitrification capacity and potential in a shallow vadose zone soil and in surficial groundwater were studied in two large blocks of a citrus grove of ‘Valencia’ orange trees (Citrus sinensis (L.) Obs.) on Rough lemon rootstock ( Citrus jambhiri (L.)) under a uniform N rate and irrigation program. The NO3–N concentration in the surficial groundwater sampled from four monitoring wells (MW) within each block varied from 5.5- to 6.6-fold. Soil samples were collected from 0 to 30, 30 to 90, or 90 to 150 cm depths, and from the soil/groundwater interface (SGWI). Groundwater samples from the monitoring wells (MW) were collected prior to purging (stagnant water) and after purging five well volumes. Without the addition of either C or N, the denitrification capacity ranged from 0.5 to 1.53, and from 0.0 to 2.25 mg N2O–N kg−1 soil at the surface soil and at the soil/groundwater interface, respectively. The denitrification potential increased by 100-fold with the addition of 200 mg kg−1 each of N and C. The denitrification potential in the groundwater also followed a pattern similar to that for the soil samples. Denitrification potential in the soil or in the groundwater was greatest near the monitor well with shallow depth of vadose zone (MW3). Cumulative N2O–N emission (denitrification capacity) from the SGWI soil samples and from stagnant water samples strongly correlated to microbial most probable number (MPN) counts (r2 = 0.84 – 0.89), and dissolved organic C (DOC) (r2 = 0.96 – 0.97). Denitrification capacity of the SGWI samples moderately correlated to water-filled pore space (WFPS) (r2 = 0.52). However, extractable NO3-N content of the SGWI soil samples poorly (negative) correlated to denitrification capacity (r2 = 0.35). However, addition C, N or both to the soil or water samples resulted in significant increase in cumulative N2O emission. This study demonstrated that variation in denitrification capacity, as a result of differences in denitrifier population, and the amount of readily available carbon source significantly (at 95% probability level) influenced the variation in NO3–N concentrations in the surficial groundwater samples collected from different monitoring wells within an area with uniform N management.
Similar content being viewed by others
References
Alexander M and Clark F E 1965 Nitrifying Bacteria. In Methods of Soil Analysis, Part 2. Eds. C A Black et al. pp 1477–1483. ASA, Madison, WI.
Alexander M 1965 Denitrifying Bacteria. In Methods of Soil Analysis, Part 2. Eds. C A Black et al. pp 1484–1486. ASA, Madison, WI.
Alpkem Corporation 1986 RFA methodology for nitrate-nitrogen. A303-S170. Alpkem Corporation, Clackamas, OR.
Alpkem Corporation 1989 RFA methodology for ammonia nitrogen. A303-S020. Alpkem Corporation, Clackamas, OR.
Alva A K and Paramasivam S 1998a Effects of monitor well purging technique on selected chemical properties of surficial groundwater. Bull. Environ. Contamin. Toxic. 60, 525–530.
Alva A K and Paramasivam S 1998b Nitrogen management for high yield and quality of citrus in sandy soils. Soil Sci. Soc. Am. J. 62, 1335–1342.
Alva A K, Paramasivam S and Graham W D 1998 Impact of nitrogen best management practice on groundwater nitrate, leaf nutritional status and yield of Valencia orange trees in a vulnerable soil. J. Environ. Qual. 27, 904–910.
Bianchi A, Garcin J and Rault P 1986 Recherches sur la microflore et les activités dénitrifiantes dans les nappes. Rapport Ministère de l=Environnement SRETE 4353, Paris. 28 p.
Burton D L and Beauchamp E G 1985 Denitrification rate relationships with soil parameters in the field. Comm. Soil Sci. Plant Anal. 16, 539–549
Carlisle V W, Sodek F, Collins M E, Hammond L C and Harris W G 1989 Characterization data for selected Florida soils. Univer sity of Florida, Institute of Food and Agricultural Sciences. Soil Science Report No. 89-1.
Christensen S 1985 Denitrification in a sandy loam as influenced by climatic and soil conditions. Tidsakr. Planteavl 89, 351–365.
Firestone M K 1984 Biological denitrification In Nitrogen in Agricultural Soils (Ed) F J Stevenson. pp 289–326. Agron. Monogr. 22. ASA, CSSA, and SSSA, Madison, WI.
Graham W D and Alva A K 1993 Annual Progress Report. Ridge Citrus water Quality Project. University of Florida, Institute of Food and Agricultural Sciences, Gainesville, FL.
Groffman P M and Tiedje J M 1989 Denitrification in north temperate forest soils: Spatial and temporal patterns at the land scape and seasonal scales. Soil Biol. Biochem. 21, 613–620.
Jones GW, DeHaven E C, Clark L F, Rauch J T, Rasmussen J R and Guillen G C 1990 Groundwater quality sampling results from wells in the Southwest Florida Water Management District. Ambient Groundwater Quality Monitoring Program, SWFWMD, and Florida Department of Environmental Regulation. Tampa, FL.
Knowles R 1982 Denitrification. Microbiol. Rev. 46(1), 43–70.
Korom S F 1991 Denitrification, in the unconsolidated deposits of the Heber Valley Aquifer, Ph.D. Thesis, Utah State Univ., Logan, UT.
Linn D M and Doran J W 1984 Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 48, 1267–1272.
Mariotti A, Landreau A and Simon B 1988 15N isotope biogeochemistry and natural denitrification process in groundwater: Application to the chalk aquifer of northern France. Geochim. Cosmochim. Acta 52(7), 1869–1879.
Martin K, Parsons L L, Murray R E and Smith M S 1988 Dynamics of soil denitrifier populations: Relationships between enzyme activity, most-probable number counts, and actual N gas loss. Appl. Environ. Microbiol. 55, 717–721.
Murray R E, Feig Y S and Tiedje J M 1995 Spatial heterogeneity in the distribution of denitrifying bacteria associated with denitrification activity zones. Appl. Environ. Microbiol. 61, 2791–2793.
Myrold D D 1988 Denitrification in ryegrass and winter wheat cropping systems of western Oregon. Soil Sci. Soc. Am. J. 52, 412–416.
National Research Council 1978 Nitrates: An environmental assessment. Natl. Acad. Sci., Washington, DC.
Obenhuber D C and Lowrance R 1991 Reduction of nitrate in aquifer microcosms by carbon additions. J. Environ. Qual. 20, 255–258.
Parkin T B 1987 Soil microsites as a source of denitrification variability. Soil Sci. Soc. Am. J. 51, 1194–1199.
Parkin T B and Robinson J A 1989 Stochastic models of soil denitrification. Appl. Environ. Microbiol. 55, 72–77.
Parsons L L, Murray R E and Smith M S 1991 Soil denitrification dynamics: Spatial and temporal variations of enzyme activity, populations, and nitrogen gas loss. Soil Sci. Soc. Am. J. 55, 90–95.
Royal Society Study Group 1983 The nitrogen cycle of the United Kingdom, p. 264. The Royal Society, London 264 p.
SAS Institute 1988 User's guide. Version 6.03 ed. SAS Inst., Cary, NC.
Slater J M and Capone D G 1987 Denitrification in aquifer soil and near shore marine sediments influenced by groundwater nitrate. Appl. Environ. Microbiol. 53, 1292–1297.
Smith R L and Duff J H 1988 Denitrification in a sand and gravel aquifer. Appl. Environ. Microbiol. 54, 1071–1078.
Smith M S and Parsons L L 1985 Persistence of denitrifying enzyme activity in dried soils. Appl. Environ. Microbiol. 49, 316–320.
Tiedje J M 1982 Denitrification. In Methods of Soil Analysis. Part 2, 2nd edn. Ed. A L Page. Am. Soc. Agron., Madison, WI.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paramasivam, S., Alva, A.K., Prakash, O. et al. Denitrification in the vadose zone and in surficial groundwater of a sandy entisol with citrus production. Plant and Soil 208, 307–319 (1999). https://doi.org/10.1023/A:1004519913339
Issue Date:
DOI: https://doi.org/10.1023/A:1004519913339