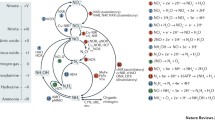
Abstract
Some aspects of inorganic nitrogen conversion by microorganisms like N2O emission and hydroxylamine metabolism studied by Beijerinck and Kluyver, founders of the Delft School of Microbiology, are still actual today. In the Kluyver Laboratory for Biotechnology, microbial conversion of nitrogen compounds is still a central research theme. In recent years a range of new microbial processes and process technological applications have been studied. This paper gives a review of these developments including, aerobic denitrification, anaerobic ammonium oxidation, heterotrophic nitrification, and formation of intermediates (NO2-, NO, N2O), as well as the way these processes are controlled at the genetic and enzyme level.
Similar content being viewed by others
References
Amann RI, Ludwig W & Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microb. Rev. 59: 143–169
Anderson KK & Hooper AB (1983) O2 and H2O are each the source of one O in NO2 − produced from NH3 by Nitrosomonas. FEBS Lett. 164: 236–240
Andrew CR, Han J, de Vries S, van der Oost J, Averill BA, Loehr ThM & Sanders-Loehr J (1994) CuA of Cytochrome c Oxidase and the A site of N2O reductase are Tetrahedrally Distorted Type I Cu Cysteinates. J. Am. Chem. Soc. 116: 10805–10806
Antholine WE, Kastrau DHW, Steffens GCM, Buse G, Zumft WG & Kroneck PMH (1992) A comparative EPR investigation of the multicopper proteins nitrous-oxide reductase and cytochrome c oxidase. Eur. J. Biochem. 209: 875–881
Anthonissen AC, Loehr RC, Prakasam TBS & Shrinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. J. Wat. Poll. Control Fed. 48: 835–852
Arciero DM & Hooper AB (1993) Hydroxylamine oxidoreductase from Nitrosomonas europaea is a multimer of an octa-heme subunit. J. Biol. Chem. 268: 14645–14654
Arciero DM, Hooper AB, Cai M & Timkovich R (1993) Evidence for the structure of the active site heme P460 in hydroxylamine oxidoreductase of Nitrosomonas. Biochemistry 32: 9370–9378
Arts PAM, Robertson LA & Kuenen JG (1995) Nitrification and denitrification by Thiosphaera pantotropha in aerobic chemostat cultures. FEMS Microbiol Ecol. 18: 305–315.
Baker SC, Goodhew CF, Thompson IP, Bramwell PA, Pettigrew G & Ferguson SJ (1995) A study of Paracoccus denitrificans using fatty acid methyl ester analysis and cytochromes c550 amino acid sequence. Proc. Beijerinck centennial (Scheffers WA & van Dijken JP Eds), pp 311–312, Delft University press, Delft, the Netherlands.
Bell LC, Richardson DJ, & Ferguson SJ (1990) Periplasmic and membrane-bound nitrate reductase in Thiosphaera pantothropa. FEBS Lett 265: 85–87.
Bell LC, Page MD, Berks BC, Richardson DJ & Ferguson SJ (1993) Insertion of transposon Tn5 into a structural gene of the membrane-bound nitrate reudctase of Thiosphaera pantothropa results in anaerobic overexpression of periplasmic nitrate reductase activity. J. Gen. Microbiol. 139: 3205–3214
Belser RT & Schmidt EL (1978) Serological diversity within a terrestrial ammonia-oxidizing population. Appl. Environ. Microbiol. 36: 589–593
Berks BC, Richardson DJ, Robinson C, Reilly A, Aplin RT & Ferguson SJ (1994) Purification and characterization of the periplasmic nitrate reductase from Thiosphaera pantotropha. Eur. J. Biochem. 220: 117–124
Berks BC, Richardson DJ, Reilly A, Willis AC & Ferguson (1995) The napEDABC gene cluster encoding the periplasmic nitrate reductase system of Thiosphaera pantotropha. Biochem. J. 309: 983–992
Bergmann DJ & Hooper AB (1994) Sequence of the gene, amoB, for the 43 kDa polypeptide of ammonia monooxygenase of Nitrosomonas europaea. Biochim Biophys res comm. 204(2): 759–762
Bergmann DJ, Arciero DM & Hooper AB (1994) Organization of the hao gene cluster of Nitrosomonas europaea: genes for two tetra heme c cytochromes. J. Bacteriol. 176: 3148–3153
Beijerinck MW (1904) De invloed der mikroben op de vruchtbaarheid van den grond en op den groei der hoogere planten. Landbouwkundig tijdschrift: 225–250.
Beijerinck MW & Minkman DCJ (1910) Bildung und Verbrauch von Stickoxydul durch Bakterien. Centr. Blatt. Bacteriol. Parasit. Jena II, 25: 30–63.
Blackburn NJ, Barr ME, Woodruff WH, van der Oost J & de Vries S (1994) Metal-Metal Bonding in Biology: EXAFS Evidence for a 2.5 β Copper-Copper Bond in the CuA Center of Cytochrome Oxidase. Biochem. 33: 10401–10407
Bock E, Koops HP, Ahlers B & Harms H (1992) Oxidation of inorganic nitrogen compounds as energy source. In: Balows A, Trueper HG, Dworkin M, Harder W, & Schleiffer KH (Eds) The Proaryotes (pp 414–430) Springer Verlag, Berlin.
Bock E, Schmidt I, Stueven R & Zart D (1995). Nitrogen loss caused by denitrifying Nitrosomonas cells using ammonium or hydrogen as electron donors and nitrite as electron acceptor. Arch. Microbiol. 163: 16–20.
Boettcher B & Koops HP (1994) Growth of lithotrophic ammonia-oxidizing bacteria on hydroxylamine. FEMS Microbiol. Lett. 122: 263–266
Breal E (1892) De la presence, dans la paille, d'un ferment aerobie, reducteur des nitrates. Compte Rendu Acad. Sci. 114: 681–684
Broda E (1977) Two kinds of lithotrophs missing in nature. Z. Allg. Mikrobiol. 17: 491–493
Brouwer M (1995) Stikstofverwijdering uit rejectie water met behulp van het SHARON proces. BSDL Thesis, Delft University of Technology.
Carr GJ & Ferguson SJ (1990) The nitric oxide reductase of Paracoccus denitrificans. Biochem. J. 269: 423–429
Carter JP, Hsiao YH, Spiro S & Richardson DJ (1995) Soil and sediment bacteria capable of aerobic nitrate respiration Appl. Environ. Microbiol. 61: 2852–2858
Craske A & Ferguson SJ (1986) The respiratory nitrate reductase from Paracoccus denitrificans. Molecular characterization and kinetic properties. Eur. J. Biochem. 158: 429–436
de Boer APN, Reijnders WNM, Kuenen JG, Stouthamer AH and van Spanning RJM (1994) Isolation, sequencing and mutational analysis of a gene cluster involved in nitrite reduction in Paracoccus denitrificans. Ant. van Leeuwenhoek 66: 111–127
de Bruijn P, van de Graaf AA, Jetten MSM, Robertson LA & Kuenen JG (1995) Growth of Nitrosomonas europaea on hydroxylamine. FEMS Microbiol. Lett. 125: 179–184
Degrange V & Bardin R (1995) Detection and Counting of Nitrobacter populations in soil by PCR. Appl. Environ. Microbiol. 61: 2093–2098
Dooley DM, McGuirl MA, Rosenzweig AC, Landin JA, Scott RA, Zumft WG, Devlin F & Stephens PhJ (1991) Spectroscopic studies of the Copper Sites in Wild-Type Pseudomonas stutzeri N2O reductase and in an Inactive Protein Isolated from a Mutant Deficient in Copper-Site Biosynthesis. Inorg. Chem. 30: 3006–3011
Ehrich S, Behrens D, Lebdeva E, Ludwig W & Bock E (1995) A new obligately chemolithoautotrophic, nitrite-oxidizing bacterium, Nitrospira moscoviensis sp. nov. and its phylogenetic relationship. Arch. Microbiol. 164: 16–23
Farrar JA, Thompson AJ, Cheesman MR, Dooley DM & Zumft WG (1991) A model of the copper centers of nitrous oxide reductase (Pseudomonas stutzeri). FEBS Lett. 294: 11–15
Ferguson SJ (1994) Denitrification and its control. A van Leeuwenhoek 66: 89–110.
Fliermans CB, Bohlool BB & Schmidt EL (1974) Autoecological study of the chemoautotroph Nitrobacter by immunofluorescence. Appl. Microbiol. 27: 124–149
Fülöp V, Moir JWB, Ferguson SJ & Hajdu J (1995) The Anatomy of a Bifunctional Enzyme: Structural Basis for Reduction of Oxygen to Water and Synthesis of Nitric Oxide by Cytochrome cd1. Cell 81: 369–377
Gayon U & Dupetit G (1886) Recherches sur la reduction des nitrates par les infinement petits. Mem Soc. Sci. Phys. Nat. Bordeaux Ser 3, 2: 201–307
Glockner AB, Jungst A & Zumft WG (1993) Copper-containing nitrite reductase from Pseudomonas aureofaciens is functional in a mutationally cytochrome cd1-free background (NirS−) of Pseudomonas stutzeri. Arch. Microbiol. 160: 18–26
Godden JW, Turley S, Teller DC, Adman ET, Liu MY, Payne WJ & LeGall J (1991) The 2.3 Angstrom X-ray Structure of Nitrite Reductase from Achromobacter cycloclastes. Science 253: 438–442
Goretski J, Zafiriou OC & Hollocher TC (1990) Steady-state Nitric Oxide Concentrations during Denitrification. J. Biol. Chem. 265: 11535–11538
Head IM, Hiorns WD, Embley TM, McCarthy AJ, and Saunders JR (1993) The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J. Gen. Microbiol. 139: 1147–1153
Heijnen JJ, Van Loosdrecht MCM, Mulder R, Weltevrede R & Mulder A (1990). Development and scale-up of an aerobic airlift suspension reactor. Water Sci. Techn. 27: 253–261
Heiss B, Frunzke K & Zumft WG (1989) Formation of the N-N bond from nitric oxide by a membrane-bound cytochrome bc complex of nitrate-respiring (denitrifying) Pseudomonas stutzeri. J. Bact. 171: 3288–3297
Hendrich MP, Logan M, Andersson KA, Arciero DM, Lipscomb JD & Hooper AB (1994) The active site of hydroxylamine oxidoreductase from Nitrosomonas: evidence for a new metal cluster in enzymes. J. Am. Chem. Soc. 116: 11961–11968
Hiorns WD, Hastings RC, Head IM, McCarthy AJ, Saunders JR, Pickup RW & Hall GG (1995) Amplification of 16 S RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of nitrosospiras in the environment. Microbiology 141: 2793–2800
Hooper AB, DiSpirito AA, Olson TC, Anderson KK, Cunningham W & Taaffe H (1984) Generation of a proton gradient by a periplamic dehydrogenase. In: Crawford RL & Hanson RS (Eds.) Microbial growth on C1 compounds (pp 53–58) American Society for Microbiology, Washington
Hunik JH, Tramper J & Wijffels RH (1994) A strategy to scale up nitrification processes with immobilized cells of Nitrosomonas europea and Nitrobacter agilis. Bioprocess Eng. II 73–82
Hyman MR, Murton IB & Arp DJ (1988) Interaction of ammonia monooxygenase with alkanes, alkenes, and alkynes. Appl. Environ. Microbiol. 54: 298–303
Iwata S, Ostermeier C, Ludwig B & Michel H (1995) Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376: 660–669
Jetten MSM, Robertson LA & Kuenen JG (1995) Recent advances in the removal of nitrogen from waste water. In: Proceedings of the International Symposium on Microbial Ecology (Tiedje JA, Ed), Brazilian Society of Microbiology, Sao Paolo, in press
Josserand A, & Cleyet-Marel JC (1979) Isolation of Nitrobacter from soils and evidence for novel serotypes using immunofluorescence. Microb. Ecol. 5: 197–205.
Juliette LY, Hyman MR & Arp DJ (1995) Roles of bovine serum albumin and copper in the assay and stability of ammonia monooxygenase activity in vitro. J. Bacteriol. 177: 4908–4913
Jungst A & Zumft WG (1992) Interdependence of respiratory NO reduction and nitrite reduction revealed by mutagenesis of nirQ, a novel gene in the denitrification gene cluster of Pseudomonas stutzeri FEBS Lett 314: 308–314
Kastrau DHW, Heiss B, Kroneck PHM & Zumft WG (1994) Nitric oxide reductase from Pseudomonas stutzeri, a novel cytochrome bc complex. Eur. J. Biochem. 222: 293–303
Kingma-Boltjes TY (1934) Onderzoekingen over nitrificeerende bacteriën. PhD thesis Delft University of Technology
Kingma-Boltjes TY (1936) Untersuchungen ueber die nitrifizierende Bakteriën. Arch. Microbiol. 6: 79–138
Klotz MG & Norton JM (1995) Sequence of an ammonia monooxygenase subunit A encoding gene from Nitrosospira sp. NpAV. Gene 163: 159–160
Kluyver AJ & Donker HJK (1926) Die Einheit in der Biochemie. Chem. Zelle u. Gewebe 13: 134–190
Kluyver AJ (1936) Bacterial Metabolism. Ann Rev Biochem 5: 539–560
Kluyver AJ (1953) Some aspects of nitrate reduction sixth international congress on microbiology. Proceedings of the symposium on microbial metabolism pp. 71–91
Kluyver AJ & Verhoeven W (1954a) Studies on true dissimilatory nitrate reduction II. The mechanism of denitrification. Anthonie van Leeuwenhoek 20: 241–262
Kluyver AJ & Verhoeven W (1954b) Studies on true dissimilatory nitrate reduction II. On the adaptation of Micrococcus denitrificans. Anthonie van Leeuwenhoek 20: 337–358
Kuenen JG & Robertson LA (1994) Combined nitrification and denitrification processes. FEMS Microbiol. Rev. 15: 109–117
Ludwig W, Mittenhuber G & Friedrich CG (1993) Transfer of Thiosphaera pantotropha to Paracoccus denitrificans. Int. J. Syst. Bacteriol. 43: 363–367
McCaig AE, Embley TM & Prosser JI (1994) Molecular analysis of enrichment cultures of marine ammonia oxidisers. FEMS Microbiology Letters 120: 363–368
McTavish H, Fuchs JA & Hooper AB (1993a) Sequence of the gene codong for ammonia monooxygenase in Nitrosomonas europaea. J. Bacteriol. 175: 2436–2444
McTavish H, Laquier F, Arciero D, Logan M, Mundform G, Fuchs JA & Hooper AB (1993b) Multiple copies of genes coding for electron transport proteins in the bacterium Nitrosomonas europaea. J. Bacteriol. 175: 2445–2447
Meiklejohn J (1940) Aerobic denitrification. An. Appl. Biol. 27: 558–573
Mulder A, van de Graaff AA, Robertson LA & Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrying fluidized bed reactor. FEMS Microbiol Ecol. 16: 177–184.
Muller EB, Stouthamer AH & Verseveld HW (1995) Simultaneous NH3 oxidation and N2 production at reduced O2 concentrations by sewage sludge subcultured with chemolithotrophic medium. Biodegadation 6: 339–349
Muyzer G, De Waal EC & Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59: 695–700
Muyzer G & Ramsing NB (1996) Molecular methods to study the organization of microbial communities. Wat. Sci. Tech. 32: 11–18
Navarro E, Simonet P, Normand P & Bardin R (1992) Characterization of natural populations of Nitrobacter spp. using PCR/RFLP analysis of the ribosomal intergenic spacer. Arch. Microbiol. 157: 107–115
Ohashi A, Viraj de Silva DG, Mobarry B, Manem JA, Stahl DA, and Rittmann BE (1996) Influence of substrate C/N ratio on the structure of multi-species biofilms consisting of nitrifiers and heterotrophs. Wat. Sci. Tech. 32: 75–84
Olsen GJ, Lane DJ, Giovannoni SJ & Pace NR (1986) Microbial ecology and evolution: a ribosomal RNA approach. Ann. Rev. Microbiol. 40: 337–365
Otte S, Grobben NG, Robertson LA, Jetten MSM & Kuenen JG (1996) N2O production by Alcaligenes faecalis during transient and dynamic aerobic and anaerobic conditions. Appl. Environ. Microbiol. in press
Rahmani H, Rols JL, Capdeville B, Cornier JC & Deguin A (1995) Nitrite removal by a fixed culture in a submerged granular biofilter. Wat. Res. 29: 1745–1753.
Richardson DJ & Ferguson SJ (1992) The influence of carbon substrate on the activity of the periplamic nitrate reductase in aerobically grown Thiosphaera pantothropa. Arch. Microbiol. 157: 535–537.
Robertson LA & Kuenen JG (1988) Heterotrophic nitrification in Thiosphaera pantotropha: Oxygen uptake and enzyme studies. J. Gen. Microbiol. 134: 857–863.
Robertson LA, van Niel EWJ, Torremans RAM & Kuenen JG (1989) Simultaneous nitrification and denitrification in aerobic chemostat cultures of Thiosphaera pantotropha. Appl. Environ. Microbiol. 540: 2812–2818
Robertson LA & Kuenen JG (1992) Nitrogen removal from water and waste. In: Microbial control of Pollution, Fry JC, Gadd GM, Herbert RA, Jones CW and Watson-Craig IA (Eds) pp 227–267. Society for General Microbiology, Reading UK.
Robertson LA, Dalsgaard T, Revsbech NP & Kuenen JG (1995) Confirmation of ‘aerobic denitrification’ in batch cultures, using gas chromatography and 15N mass spectrometry. FEMS Microbiol. Ecol. 18: 113–120
Rotthauwe JH, de Boer W & Liesack W (1995) Comparative analysis of gene sequences encoding ammonia monooxygenase of Nitrosospira sp. AHB1 and Nitrosolobus multiformis C-71. FEMS Microbiol. Lett. 133: 131–135
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491
Sanden B, Grunditz C, Hansson Y & Dalhammar D (1994) Quantification and characterisation of Nitrosomas and Nitrobacter using monoclonal antibodies. Wat. Sci. Tech. 29: 1–6.
Saraste, M (1990) Structural features of cytochrome oxidase. Quart. Rev. Biophys. 23: 331–366
Saraste, M & Castresana, J (1994) Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 341: 1–4
Saraswat N, Alleman JE & Smith TJ (1994) Enzyme Immunoassay detection of Nitrosomonas europaea. Appl. Environ. Microbiol. 60: 1969–1973
Sayavedra-Soto LA, Hommes NG & Arp DJ (1993) Characterization of the gene encoding Hydroxylamine Oxidoreductase in Nitrosomonas europaea. J. Bacteriol. 176: 504–510
Siddiqui RA, Warnecke-Eberz U, Hengsberger A, Schneider B, Kostka S & Friedrich B (1993) Structure and Function of a Periplasmic Nitrate Reductase in Alcaligenes eutrophus H16. J. Bacteriol. 175: 5867–5876
Sinigalliano CD, Kuhn DN & Jones RD (1995) Amplification of the amoA gene from diverse species of ammonium-oxidizing bacteria and from an indigenous bacterial population from seawater. Appl. Environ. Microbiol. 61: 2702–2706
Stehr G, Boettcher B, Dittberner P, Rath G & Koops HP (1995) The ammonia-oxidizing nitrifying population of the River Elbe estuary. FEMS Microbiol. Ecol. 17: 177–186
Teske A, Alm E, Regan JM, Toze S, Rittman BE & Stahl DA (1994) Evolutionary relationships among ammonia-and nitrite-oxidizing bacteria. J. Bacteriol. 176: 6623–6630
Tijhuis L, Huisman JL, Hekkelman HD, Van Loosdrecht MCM & Heijnen JJ (1995) Formation of nitrifying biofilms on small suspended particles in airlift reactors. Biotechnol. Bioeng. 31, in press
Tsukihara T. Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R & Yoshikawa S (1995) Structures of Metal Sites of Oxidized Bovine Heart Cytochrome c Oxidase at 2.8 Å. Science 269: 1069–1074
Turk O & Mavinic DS (1989) Stability of nitrite build up in an activatd sludge system. J. Wat. Poll. Control. Fed. 61: 1440–1448.
van de Graaf AA, Mulder A, Slijkhuis H, Robertson LA & Kuenen JG (1990) Anoxic ammonium oxidation. In: Proc. 5th European Congress on Biotechnology (Christiansen C, Munck L & Villadsen J Eds) vol 1, pp388–391, Munkgaard Internatinal Publishers, Copenhagen.
van de Graaf AA, Mulder A, de Bruijn P, Jetten MSM, Robertson LA & Kuenen JG (1995) Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61: 1246–1251
van de Graaf AA, de Bruijn P, Robertson LA, Jetten MSM & Kuenen JG (1996) Autotrophic growth of anaerobic, ammonium-oxidising microorgansims in a fluidized bed reactor. Microbiology, in press.
van der Oost J, de Boer APN, de Gier J-WL, Zumft WG, Stouthamer AH & van Spanning RJM (1994) The heme-copper oxidase family consists of three distinct types of terminal oxidases is related to nitric oxide reductase. FEMS Microbiol. Lett. 121: 1–10
van Loosdrecht MCM, Tijhuis L, Wijdieks AMS & Heijnen JJ (1995) Population distribution in aerobic bioflms on small suspended particles. Wat. Sci. Techn. 31: 163–171
van Niel EWJ, Arts PAM, Wesselink BJ, Robertson LA, and Kuenen JG (1993) Competition between heterotrophic and autotrophic nitrifiers for ammonia in chemostat cultures. FEMS Microbiol. Ecol. 102: 109–118
van Niel EWJ, Braber KJ, Robertson LA & Kuenen JG (1992) Heterotrophic nitrification and aerobic denitrification in Alcaligenes faecalis strain TUD. A van Leeuwenhoek 62: 231–237
van Niel CB (1949) The Delft school and the rise of general microbiology. Bact. Rev 13: 161–174
van Niel CB (1954) The chemoutotrophic and photosynthetic bacteria. Ann. Rev. Microbiol 8: 105–132
van Niel CB (1967) The education of a microbiologist. Ann. Rev. Microbiol. 21: 1–30
Verhoeven W (1956a) Some remarks on nitrate and nitrite metabolism in microorganisms. In Inorganic nitrogen metabolism McElroy WD and Glass B (Eds) John Hopkins Press Baltimore pp 61–83
Verhoeven W (1956b) Sudies on true dissimilatory nitrate reduction V. Nitric oxide production and consumption by microorgansims. A van Leeuwenhoek 22: 385–406
Verstraete W (1975) Heterotrophic nitrification in soils and aqueous media. Izvestija Akad. Nauk. SSSR Ser. Biol. 4: 541–558
Von Wachenfeldt C, de Vries S & van der Oost J (1994) The CuA site of the caa3-type oxidase of Bacillus subtilis is a mixed-valence binuclear copper center. FEBS Lett. 340: 109–113
Voytek MA & Ward BB (1995) Detection of ammonium-oxidizing bacteria of the beta-subclass of the class Proteobacteria in aquatic samples with PCR. Appl. Environ. Microbiol. 61: 1444–1450.
Wagner M, Rath G, Amann R, Koops HP & Schleifer KH (1995) In situ indentification of ammonia-oxidizing bacteria. System. Appl. Microbiol. 18: 251–264
Ward BB & Perry MJ (1980) Immunofluorescent assay for the marine ammonium-oxidizing bacterium Nitrosococcus oceanus. Appl. Environ. Microbiol. 39: 913–918
Ward BB (1982) Oceanic distribution of ammonium-oxidizing bacteria determined by immunofluorescent assay. J. Mar. Res. 40: 1155–1172.
Ward BB & Carlucci AF (1985) Marine ammonia-and nitrite-oxidizing bacteria: serological diversity determined by immunofluorescence in culture and in the environment. Appl. Environ. Microbiol. 50: 194–201
Watson SW, Bock E, Harms H, Koops HP & Hooper AB (1989) Nitrifying bacteria. In: Holt JC, Staley JT, Bryant MP & Pfennig N (eds) Bergey's manual of systematic bacteriology vol. 3 pp. 1808–1834, Williams and Wilkins, Baltimore.
Wehrfritz JM, Reilly A, Spiro S & Richarson DJ (1993) Purification of hydroxylamine oxidase from Thiosphaera pantotropha. FEBS Lett. 335: 246–250
Winogradsky S (1890) Recherches sur le organismes de la nitrification. Ann. Inst. Pasteur 4: 213–231
Wood PM (1986) Nitrifcation as a bacterial energy source. In: Nitrification, Prosser JI (ed) pp. 39–62, IRL Press, Oxford UK.
Ye RW, Averill BA & Tiedje, JM (1994) Denitrification: Production & consumption of nitric oxide. Appl. & Environm. Microbiol. 60: 1053–1058
Yoshioka T, Hisayoshi H & Saijo Y (1982) Growth kinetic studies of nitrifying bacteria by immunofluorescent counting method. Appl. Microbiol. 28: 169–180
Zumft WG (1994) The biological role of nitric oxide in bacteria. Arch. Microbiol. 160: 253–264
Zumft WG, Braun C & Cuypers, H (1994) Nitric oxide reductase from Pseudomonas stutzeri Primary structure & gene organization of a novel bacterial cytochrome bc complex. Eur. J. Biochem. 219: 481–490
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jetten, M.S., Logemann, S., Muyzer, G. et al. Novel principles in the microbial conversion of nitrogen compounds. Antonie Van Leeuwenhoek 71, 75–93 (1997). https://doi.org/10.1023/A:1000150219937
Issue Date:
DOI: https://doi.org/10.1023/A:1000150219937