Abstract
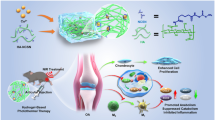
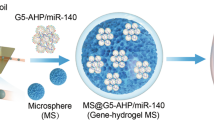
This paper presents a new method of delivering shRNA with biodegradable, thermosensitive PLGA-PEG-PLGA hydrogels for gene treatment of osteoarthritis (OA). OA is a chronic debilitating disease. Without the proper treatment and prognosis, it may result in the loss of joint function in aged people. Currently, gene therapy targeted on WISP-1 has emerged as an alternative method for OA treatment. In order to constantly release shRNA at 37.0 °C, we synthetized the hydrogels via ring-opening copolymerization of lactide (LA) and glycolide (GA) using Polyethylene glycol (PEG Mn = 1000) and stannous octoate (Sn(Oct)2, 95%) as the macroinitiator and catalyst. First, the PLGA-PEG-PLGA copolymer was mixed with WISP-1shRNA and PEI-Lys in distilled water at 4.0 °C. Then, the WISP-1shRNA/PEI-Lys loaded hydrogel was formed after incubation of the mixed solution at 37.0 °C. During tests, the plasmid was released from this hydrogel complex constantly, and enhanced the transfection efficiency of WISP-1shRNA. In addition, silencing WISP-1 results to lower expression of MMP-3 and ADAMTS, and the accumulation of HBP1 in synoviocytes. Therefore, the hydrogel containing WISP-1shRNA is demonstrated an efficient way for the treatment of OA.
Similar content being viewed by others
References
Goldring M B, Goldring S R. Osteoarthritis. Journal of Cellular Physiology, 2007, 213, 626–634.
Wieland H A, Michaelis M, Kirschbaum B J, Rudolphi K A. Osteoarthritis - an untreatable disease? Nature Reviews Drug Discovery, 2005, 4, 331–344.
Vavken P, Dorotka R. Burden of musculoskeletal disease and its determination by urbanicity, socioeconomic status, age, and sex: Results from 14,507 subjects. Arthritis Care & Research, 2011, 63, 1558–1564.
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain, 2006, 10, 287–333.
Loza E, Abasolo L, Jover J A, Carmona L. Burden of disease across chronic diseases: A health survey that measured prevalence, function, and quality of life. The Journal of Rheumatology, 2008, 35, 159–165.
Conaghan P G, Kloppenburg M, Schett G, Bijlsma J W. Osteoarthritis research priorities: a report from a EULAR ad hoc expert committee. Annals of the Rheumatic Diseases, 2014, 73, 1442–1445.
Loza E, Lopez-Gomez J M, Abasolo L, Maese J, Carmona L, Batlle-Gualda E. Economic burden of knee and hip osteoarthritis in Spain. Arthritis and Rheumatism, 2009, 61, 158–165.
Jevsevar D S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. Journal of the American Academy of Orthopaedic Surgeons, 2013, 21, 571–576.
Fernandes L, Hagen K B, Bijlsma J W J, Andreassen O, Christensen P, Conaghan P G, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander L S, Lund H, Mallen C D, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silva J A, de la Torre J, Zanoli G, Vliet Vlieland T P M. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Annals of the Rheumatic Diseases, 2013, 72, 1125–1135.
Bijlsma J W, Berenbaum F, Lafeber F P. Osteoarthritis: An update with relevance for clinical practice. Lancet, 2011, 377, 2115–2126.
Smith E, Hoy D, Cross M, Merriman T R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases, 2014, 73, 1323–1330.
Spector T D, MacGregor A J. Risk factors for osteoarthritis: Genetics. Osteoarthritis and Cartilage, 2004, 12(Suppl A): S39–S44.
Miller R E, Lu Y, Tortorella M D, Malfait A M. Genetically engineered mouse models reveal the importance of proteases as osteoarthritis drug targets. Current Rheumatology Reports, 2013, 15, 350.
Li N G, Shi Z H, Tang Y P, Wang Z J, Song S L, Qian L H, Qian D W, Duan J A. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Current Medicinal Chemistry, 2011, 18, 977–1001.
Liu F, Kohlmeier S, Wang C Y. Wnt signaling and skeletal development. Cellular Signalling, 2008, 20, 999–1009.
Studer D, Millan C, Ozturk E, Maniura-Weber K, Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. European Cells & Materials, 2012, 24, 118–135.
Lories R J, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas J T, Luyten F P. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis & Rheumatism, 2007, 56, 4095–4103.
Blom A B, Brockbank S M, van Lent P L, van Beuningen H M, Geurts J, Takahashi N, van der Kraan P M, van de Loo F A, Schreurs B W, Clements K, Newham P, van den Berg W B. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: Prominent role of Wnt-induced signaling protein 1. Arthritis & Rheumatism, 2009, 60, 501–512.
Hannon G J. RNA interference. Nature, 2002, 418, 244–251.
Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 1998, 391, 806–811.
Mittal V. Improving the efficiency of RNA interference in mammals. Nature Reviews Genetics, 2004, 5, 355–365.
van Osch G J. Osteoarthritis year in review 2014: Highlighting innovations in basic research and clinical applications in regenerative medicine. Osteoarthritis & Cartilage, 2014, 22, 2013–2016.
Ma H, He C, Cheng Y, Li D, Gong Y, Liu J, Tian H, Chen X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials, 2014, 35, 8723–8734.
He C, Zhuang X, Tang Z, Tian H, Chen X. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Advanced Healthcare Materials, 2012, 1, 48–78.
Tian H, Lin L, Jiao Z, Guo Z, Chen J, Gao S, Zhu X, Chen X. Polylysine-modified polyethylenimine inducing tumor apoptosis as an efficient gene carrier. Journal of Controlled Release, 2013, 172, 410–418.
Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chemical Society Reviews, 2008, 37, 1473–1481.
Jeong J H, Kim S W, Park T G. Biodegradable triblock copolymer of PLGA-PEG-PLGA enhances gene transfection efficiency. Pharmaceutical Research, 2004, 21, 50–54.
Langer R, Tirrell D A. Designing materials for biology and medicine. Nature, 2004, 428, 487–492.
Moffatt S, Cristiano R J. Uptake characteristics of NGR-coupled stealth PEI/pDNA nanoparticles loaded with PLGA-PEG-PLGA tri-block copolymer for targeted delivery to human monocyte-derived dendritic cells. International Journal of Pharmaceutics, 2006, 321, 143–154.
Ta H T, Dass C R, Larson I, Choong P F, Dunstan D E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials, 2009, 30, 4815–4823.
Li Z, Ning W, Wang J, Choi A, Lee P Y, Tyagi P, Huang L. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharmaceutical Research, 2003, 20, 884–888.
Lee P Y, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-β1 gene delivery vehicle enhances diabetic wound healing. Pharmaceutical Research, 2003, 20, 1995–2000.
Huang Y C, Chiang C Y, Li C H, Chang T C, Chiang C S, Chau L K, Huang K W, Wu C W, Wang S C, Lyu S R. Quantification of tumor necrosis factor-α and matrix metalloproteinases-3 in synovial fluid by a fiber-optic particle plasmon resonance sensor. Analyst, 2013, 138, 4599–4606.
Wang H S, Kuo P Y, Yang C C, Lyu S R. Matrix metalloprotease-3 expression in the medial plica and pannus-like tissue in knees from patients with medial compartment osteoarthritis. Histopathology, 2011, 58, 593–600.
Bondeson J, Blom A B, Wainwright S, Hughes C, Caterson B, van den Berg W B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis & Rheumatism, 2010, 62, 647–657.
Wang Z, Luo J, Iwamoto S, Chen Q. Matrilin-2 is proteolytically cleaved by ADAMTS-4 and ADAMTS-5. Molecules (Basel, Switzerland), 2014, 19, 8472–8487.
Durham T B, Klimkowski V J, Rito C J, Marimuthu J, Toth J L, Liu C, Durbin J D, Stout S L, Adams L, Swearingen C, Lin C, Chambers M G, Thirunavukkarasu K, Wiley M R. Identification of potent and selective hydantoin inhibitors of Aggrecanase-1 and Aggrecanase-2 that are efficacious in both chemical and surgical models of osteoarthritis. Journal of Medicinal Chemistry, 2014, 57, 10476–10485.
Majumdar M K, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, Morris E A, Glasson S S. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis & Rheumatism, 2007, 56, 3670–3674.
Blom A B, van Lent P L, van der Kraan P M, van den Berg W B. To seek shelter from the WNT in osteoarthritis? WNT-signaling as a target for osteoarthritis therapy. Current Drug Targets, 2010, 11, 620–629.
Nakamura Y, Nawata M, Wakitani S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. The American Journal of Pathology, 2005, 167, 97–105.
Alcaraz M J, Megias J, Garcia-Arnandis I, Clerigues V, Guillen M I. New molecular targets for the treatment of osteoarthritis. Biochemical Pharmacology, 2010, 80, 13–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yubao, G., Hecheng, M. & Jianguo, L. Controlled WISP-1 shRNA Delivery Using Thermosensitive Biodegradable Hydrogel in the Treatment of Osteoarthritis. J Bionic Eng 12, 285–293 (2015). https://doi.org/10.1016/S1672-6529(14)60121-9
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1672-6529(14)60121-9