Abstract
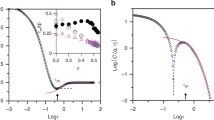
The finite autocorrelation time of thermal noise is crucial to unidirectional transportation on the molecular scale. Therefore, it is important to understand the cause of the intrinsic picosecond autocorrelation time of thermal noise in water. In this work, we use molecular dynamics simulations to compare the autocorrelation behaviors of the thermal noise, hydrogen bonds, and molecular rotations found in water. We found that the intrinsic picosecond autocorrelation time for thermal noise is caused by finite molecular rotation relaxation, in which hydrogen bonds play the role of a bridge. Furthermore, the simulation results show that our method of calculating the autocorrelation of thermal noise, by observing the fluctuating force on an oxygen atom of water, provides additional information about molecular rotations. Our findings may advance the understanding of the anomalous dynamic nanoscale behavior of particles, and the applications of terahertz technology in measuring the structural and dynamical information of molecules in solutions.





Similar content being viewed by others
References
A. Efremov, Z. Wang, Universal optimal working cycles of molecular motors. Phys. Chem. Chem. Phys. 13, 6223–6233 (2011). https://doi.org/10.1039/C0CP02118K
M. Alvarez-Pérez, S.M. Goldup, D.A. Leigh et al., A chemically-driven molecular information ratchet. J. Am. Chem. Soc. 130, 1836–1838 (2008). https://doi.org/10.1021/ja7102394
A. Yildiz, P.R. Selvin, Fluorescence imaging with one nanometer accuracy: application to molecular motors. Acc. Chem. Res. 38, 574–582 (2005). https://doi.org/10.1021/ar040136s
T.E. Kelly, R.A. Silva, H.D. Silva et al., A rationally designed prototype of a molecular motor. J. Am. Chem. Soc. 122, 6935–6949 (2000). https://doi.org/10.1021/ja001048f
B. Wand, L. Vuković, P. Král, Nanoscale rotary motors driven by electron tunneling. Phys. Rev. Lett. 101, 186808 (2008). https://doi.org/10.1103/PhysRevLett.101.186808
J. Liu, G. Shi, P. Guo et al., Blockage of water flow in carbon nanotubes by ions due to interactions between cations and aromatic rings. Phys. Rev. Lett. 115, 164502 (2015). https://doi.org/10.1103/PhysRevLett.115.164502
F. Ganazzoli, G. Raffaini, Computer simulation of polypeptideadsorption on model biomaterials. Phys. Chem. Chem. Phys. 7, 3651–3663 (2005). https://doi.org/10.1039/B506813D
J. Su, K. Yang, H. Guo, Asymmetric transport of water molecules through a hydrophobic conical channel. RSC Adv. 4, 40193–40198 (2014). https://doi.org/10.1039/C4RA07034H
E.R. Cruz-Chu, A. Aksimentiev, K. Schulten, Ionic current rectification through silica nanopores. J. Phys. Chem. C 113, 1850–1862 (2009). https://doi.org/10.1021/jp804724p
Y. Wand, Y. Zhao, J. Huang, Giant pumping of single-file water molecules in a carbon nanotube. J. Phys. Chem. B 115, 13275–13279 (2011). https://doi.org/10.1021/jp2069557
C. Zhu, H. Li, S. Meng, Transport behavior of water molecules through two-dimensional nanopores. J. Chem. Phys. 141, 18C528 (2014). https://doi.org/10.1063/1.4898075
X. Nan, Y.W. Guo, R.Z. Wan, Effect of Na and Cl ions on water evaporation on graphene oxide. Nucl. Sci. Tech. 30, 122 (2019). https://doi.org/10.1007/s41365-019-0646-7
C. Caleman, D. van der Spoel, Evaporation from water clusters containing singly charged ions. Phys. Chem. Chem. Phys. 9, 5105–5111 (2007). https://doi.org/10.1039/B706243E
R. Levy, M. Maaloum, Measuring the spring constant of atomic force microscope cantilevers: thermal fluctuations and other methods. Nanotechmology 13, 33 (2002). https://doi.org/10.1088/0957-4484/13/1/307
A. Burtzlaff, A. Weismann, M. Brandbyge et al., Shot noise as a probe of spin-polarized transport through single atoms. Phys. Rev. Lett. 114, 016602 (2015). https://doi.org/10.1103/PhysRevLett.114.016602
Y. Hovav, I. Kaminker, D. Shimon et al., The electron depolarization during dynamic nuclear polarization: measurements and simulations. Phys. Chem. Chem. Phys. 17, 226–244 (2015). https://doi.org/10.1039/C4CP03825H
H.J. Butt, M. Jaschke, Calculation of thermal noise in atomic force microscopy. Nanotechmology 6, 1 (1995). https://doi.org/10.1088/0957-4484/6/1/001
J. Siódmiak, P. Beldowski, Hyaluronic acid dynamics and its interaction with synovial fluid components as a source of the color noise. Fluct. Noise Lett. 18, 1940013 (2019). https://doi.org/10.1142/S0219477519400133
P. Hanggi, P. Jung, Colored noise in dynamical systems. Adv. Chem. Phys. 89, 239–326 (1995). https://doi.org/10.1002/9780470141489.ch4
X.J. Gong, J.Y. Li, H.J. Lu et al., A charge-driven molecular water pump. Nat. Nanotechnol. 2, 709–712 (2007). https://doi.org/10.1038/nnano.2007.320
S. Joseph, N.R. Aluru, Pumping of confined water in carbon nanotubes by rotation–translation coupling. Phys. Rev. Lett. 101, 064502 (2008). https://doi.org/10.1103/PhysRevLett.101.064502
F. Detcheverry, L. Bocquet, Thermal fluctuations in nanofluidic transport. Phys. Rev. Lett. 109, 024501 (2012). https://doi.org/10.1103/PhysRevLett.109.024501
D.J. Bonthuis, K. Falk, C.N. Kaplan et al., Comment on “pumping of confined water in carbon nanotubes by rotation–translation coupling”. Phys. Rev. Lett. 105, 209401 (2010). https://doi.org/10.1103/PhysRevLett.105.209401
D.J. Bonthuis, D. Horinek, L. Bocquet et al., Electrokinetics at aqueous interfaces without mobile charges. Langmuir 26, 12614–12625 (2010). https://doi.org/10.1021/la9034535
M.E. Suk, N.R. Aluru, Suk and Aluru Reply. Phys. Rev. Lett. 105, 209402 (2010). https://doi.org/10.1103/PhysRevLett.105.209402
R.Z. Wan, J. Hu, H.P. Fang, Asymmetric transportation induced by thermal noise at the nanoscale. Sci. China Phys. Mech. 55, 751–756 (2012). https://doi.org/10.1007/s11433-012-4695-8
Z. Zhu, N. Sheng, R.Z. Wan et al., Intrinsic autocorrelation time of picoseconds for thermal noise in water. J. Phys. Chem. A 118, 8936–8941 (2014). https://doi.org/10.1021/jp5009785
Z. Zhu, N. Sheng, H.P. Fang et al., Colored spectrum characteristics of thermal noise on the molecular scale. Phys. Chem. Chem. Phys. 18, 30189–30195 (2016). https://doi.org/10.1039/C6CP04433F
S. Mukherjee, S. Mondal, B. Bagchi, Mechanism of solvent control of protein dynamics. Phys. Rev. Lett. 122, 058101 (2019). https://doi.org/10.1103/PhysRevLett.122.058101
B.J. Gertner, R.M. Whitnell, K.R. Wilson et al., Activation to the transition state: reactant and solvent energy flow for a model SN2 reaction in water. J. Am. Chem. Soc. 113, 74–87 (1991). https://doi.org/10.1021/ja00001a014
J.D. Bernal, R.H. Fowler, A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1, 515–548 (1933). https://doi.org/10.1063/1.1749327
Y.W. Guo, R.Z. Wan, Evaporation of nanoscale water on a uniformly complete wetting surface at different temperatures. Phys. Chem. Chem. Phys. 20, 12272–12277 (2018). https://doi.org/10.1039/C8CP00037A
C.N. Peng, The effects of hydrogen on the helium behavior in palladium. Nucl. Sci. Tech. 27, 106 (2016). https://doi.org/10.1007/s41365-016-0115-5
W. Xu, Y.S. Tu, C.L. Wang et al., Water transport through T-shaped carbon nanotubes. Nucl. Sci. Tech. 22, 307–310 (2011). https://doi.org/10.13538/j.1001-8042/nst.22.307-310
X. Ren, B. Zhou, C. Wang, Promoting effect of ethanol on dewetting transition in the confined region of melittin tetramer. Nucl. Sci. Tech. 23, 252–256 (2012). https://doi.org/10.13538/j.1001-8042/nst.23.252-256
L.W. Gao, X.B. Xia, X.Q. Xu et al., Immobilization of radioactive fluoride waste in aluminophosphate glass: a molecular dynamics simulation. Nucl. Sci. Tech. 29, 92 (2018). https://doi.org/10.1007/s41365-018-0443-8
X.C. Nie, B. Zhou, C.L. Wang et al., Wetting behaviors of methanol, ethanol, and propanol on hydroxylated SiO2 substrate. Nucl. Sci. Tech. 29, 18 (2018). https://doi.org/10.1007/s41365-018-0364-6
C.L. Zhao, W.Z. Sun, X.D. Lv et al., Incident angle effect on F+ ions interaction with β-SiC: Molecular dynamics simulation. Nucl. Tech. 34, 1 (2011). http://d.old.wanfangdata.com.cn/Periodical/hjs201101012. (in Chinese)
Y.F. Ding, Z.B. Zhang, X.Z. Ke et al., Investigation on single carbon atom transporting through the single-walled carbon nanotube by MD simulation. Nucl. Tech. 28, 4 (2005). https://doi.org/10.3321/j.issn:0253-3219.2005.04.012. (in Chinese)
N. Sheng, Y.S. Tu, P. Guo et al., Asymmetrical free diffusion with orientation-dependence of molecules in finite timescales. Sci. China Phys. Mech. 56, 1047–1052 (2013). https://doi.org/10.1007/s11433-013-5081-x
J.Y. Su, H.X. Guo, Control of unidirectional transport of single-file water molecules through carbon nanotubes in an electric field. ACS Nano 5, 351–359 (2011). https://doi.org/10.1021/nn1014616
S. Pronk, S. Pall, R. Schulz et al., GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013). https://doi.org/10.1093/bioinformatics/btt055
W.L. Jorgensen, J. Chandrasekhar, J.D. Madura et al., Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983). https://doi.org/10.1063/1.445869
S. Nose, A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984). https://doi.org/10.1080/00268978400101201
W.G. Hoover, Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985). https://doi.org/10.1103/PhysRevA.31.1695
T. Darden, D. York, L. Pedersen, Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993). https://doi.org/10.1063/1.464397
W. Nadler, T. Krausche, Universality in hydrogen-bond networks. Phys. Rev. A 44, 7888–7890 (1991). https://doi.org/10.1103/PhysRevA.44.R7888
L.J. Zhang, W. Jian, L. Yi et al., A novel water layer structure inside nanobubbles at room temperature. Nucl. Sci. Tech. 25, 060503 (2014). https://doi.org/10.13538/j.1001-8042/nst.25.060503
M.X. He, M. Li, Z. Tian et al., Terahertz spectral properties of melamine and its deuterated isotope, melamine-d6. Nucl. Sci. Tech. 23, 209–214 (2012). https://doi.org/10.13538/j.1001-8042/nst.23.209-214
S.J. Shao, P. Guo, L. Zhao et al., Ordered water monolayer on ionic model substrates studied by molecular dynamics simulations. Nucl. Sci. Tech. 25, 020502 (2014). https://doi.org/10.13538/j.1001-8042/nst.25.020502
Z. Zhu, H.K. Guo, X.K. Jiang et al., Reversible hydrophobicity–hydrophilicity transition modulated by surface curvature. J. Phys. Chem. Lett. 9, 2346–2352 (2018). https://doi.org/10.1021/acs.jpclett.8b00749
Z. Zhu, C. Chang, Y.S. Shu et al., Transition to a superpermeation phase of confined water induced by a terahertz electromagnetic wave. J. Phys. Chem. Lett. 11, 256–262 (2019). https://doi.org/10.1021/acs.jpclett.9b03228
G. Hummer, J.C. Rasaiah, J.P. Noworyta, Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001). https://doi.org/10.1038/35102535
M. Thomas, M. Brehm, R. Fligg et al., Computing vibrational spectra from ab initio molecular dynamics. Phys. Chem. Chem. Phys. 15, 6608–6622 (2013). https://doi.org/10.1039/C3CP44302G
D.L. Gilden, Cognitive emissions of 1/f noise. Psychol. Rev. 108, 33–56 (2001). https://doi.org/10.1037/0033-295X.108.1.33
P. Stoica, R.L. Moses, Spectral Analysis of Signals (Pearson Prentice Hall, Upper Saddle River, 2005)
D.L. Gilman, T. Thornton, M.W. Mallon, 1/f noise in human cognition. Science 267, 1837–1839 (1995). https://doi.org/10.1126/science.7892611
T. Ji, H.W. Zhao, P.Y. Han et al., Terahertz identification and quantification of penicillamine enantiomers. Nucl. Sci. Tech. 24, 010201 (2013). https://doi.org/10.13538/j.1001-8042/nst.2013.01.005
Acknowledgements
We thank Prof. Hai-Ping Fang for his constructive suggestions and are grateful to Xing Liu, Xue-Chuan Nie, Gang Fang, and Yi-Zhou Yang for their help. The authors also appreciate the support received from the Shanghai Supercomputer Center of China, the Computer Network Information Center of the Chinese Academy of Science, the National Supercomputing Center in Shenzhen (Shenzhen Cloud Computing Center), and the Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (second phase).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the National Key Research and Development Program of China (No. 2018YFE0205501 and 2018YFB1801500), the National Natural Science Foundation of China (No. 11904231), the Shanghai Sailing Program (No. 19YF1434100).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, YW., Qin, JY., Hu, JH. et al. Molecular rotation-caused autocorrelation behaviors of thermal noise in water. NUCL SCI TECH 31, 53 (2020). https://doi.org/10.1007/s41365-020-00767-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-020-00767-w