Abstract
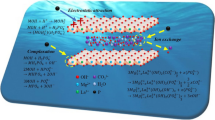
In this study, Mg–Al layered double hydroxide (MgAl-LDH) was synthesized by the urea hydrolysis method and used as sorbent to remove phosphate from aqueous solutions. Structural and morphological studies show that MgAl-LDH was prepared successfully with good crystallinity. The influences of sorbent dosage, time, initial concentrations, solution pH, temperature and competitive ions on the phosphate uptake have been investigated. It has been found that the uptake of phosphate onto MgAl-LDH was rapid and reached equilibrium within 60 min. The kinetic data were studied in terms of the pseudo-first-order, pseudo-second-order, elovich and intraparticle diffusion kinetic models. The pseudo-second-order model best described the sorption process. The isothermal data are best fitted by Freundlich, suggesting that multiple processes may control the adsorption of phosphate on the MgAl-LDH. The maximum adsorption capacity at 25 °C was found to be 346.4 mg/g. The sorption mechanism of phosphate onto LDHs could be via electrostatic attraction, ligand exchange and ion exchange. Phosphates ion uptake in the presence of other ions decreases in the order NO3− > Cl− > SO42−. Thermodynamic experiments suggested that the phosphate adsorption was spontaneous (− ΔG°) and endothermic (+ ΔH°) and increased the randomness (+ ΔS°) in the system. Thus, MgAl-LDH prepared by urea hydrolysis method is an effective sorbent for the removal of phosphate from aqueous solutions.









Similar content being viewed by others
Change history
13 March 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s41204-023-00315-y
References
Das J, Patra BS, Baliarsingh N, Parida KM (2006) Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl Clay Sci 32(3):252–260. https://doi.org/10.1016/j.clay.2006.02.005
Villalba G, Liu Y, Schroder H, Ayres RU (2008) Global phosphorus flows in the industrial economy from a production perspective. J Ind Ecol 12(4):557–569. https://doi.org/10.1111/j.1530-9290.2008.00050.x
Cheng X, Huang X, Wang X, Zhao B, Chen A, Sun D (2009) Phosphate adsorption from sewage sludge filtrate using zinc–aluminum layered double hydroxides. J Hazard Mater 169(1):958–964. https://doi.org/10.1016/j.jhazmat.2009.04.052
Cordell D, White S (2011) Peak phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3(10):2027–2049. https://doi.org/10.3390/su3102027
Scholz RW, Ulrich AE, Eilittä M, Roy A (2013) Sustainable use of phosphorus: a finite resource. Sci Total Environ 461–462:799–803. https://doi.org/10.1016/j.scitotenv.2013.05.043
Yeoman S, Stephenson T, Lester JN, Perry R (1988) The removal of phosphorus during wastewater treatment: a review. Environ Pollut 49(3):183–233. https://doi.org/10.1016/0269-7491(88)90209-6
Müller JA (2001) Prospects and problems of sludge pre-treatment processes. Water Sci Technol 44(10):121–128. https://doi.org/10.2166/wst.2001.0598
Manikandan V et al (2017) Fabrication and characterization of TiO2-loaded Moringa oleifera gum-activated carbon and the photo-catalytic degradation of phosphate in aqueous solutions. Nanotechnol Environ Eng 3(1):1. https://doi.org/10.1007/s41204-017-0031-x
Yuan J, Wen Y, Ruiz G, Sun W, Ma X (2020) Enhanced phosphorus removal and recovery by metallic nanoparticles-modified biochar. Nanotechnol Environ Eng 5(3):26. https://doi.org/10.1007/s41204-020-00090-0
Fathy M, Zayed MA, Mohamed AMG (2019) Phosphate adsorption from aqueous solutions using novel Zn Fe/Si MCM-41 magnetic nanocomposite: characterization and adsorption studies. Nanotechnol Environ Eng 4(1):14. https://doi.org/10.1007/s41204-019-0061-7
Chiban M, Soudani A, Zerbet M, Sinan F (2013) Wastewater treatment processes, Chapter 10. In: Handbook of wastewater treatment
Chiban M, Soudani A, Sinan F, Persin M (2011) Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surf B
Carja G, Popa I, Ciobanu G (2002) Mixed oxides derived from vanadium substituted layered double hydroxides. Manag J
Ouasfi N, Zbair M, Sabbar EM, Khamliche L (2019) High performance of Zn–Al–CO3 layered double hydroxide for anionic reactive blue 21 dye adsorption: kinetic, equilibrium, and thermodynamic studies. Nanotechnol Environ Eng 4(1):16. https://doi.org/10.1007/s41204-019-0063-5
Kang HR, da Fernandes CJC, da Silva RA, Constantino VRL, Koh IHJ, Zambuzzi WF (2018) Mg–Al and Zn–Al layered double hydroxides promote dynamic expression of marker genes in osteogenic differentiation by modulating mitogen-activated protein kinases. Adv Healthc Mater 7(4):1700693. https://doi.org/10.1002/adhm.201700693
Wang F, Guo Z (2019) Facile synthesis of superhydrophobic three-metal-component layered double hydroxide films on aluminum foils for highly improved corrosion inhibition. N J Chem 43(5):2289–2298. https://doi.org/10.1039/c8nj05732j
Cavani F, Trifirò F, Vaccari A (1991) Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal Today 11(2):173–301. https://doi.org/10.1016/0920-5861(91)80068-K
Zhao Y, Li F, Zhang R, Evans DG, Duan X (2002) Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem Mater 14(10):4286–4291. https://doi.org/10.1021/cm020370h
Ichou AA et al (2020) Adsorption of Pb(II) from aqueous solutions onto MgFeAl-CO3 LDH: thermodynamic and kinetic studies. DWT 178:193–202. https://doi.org/10.5004/dwt.2020.24952
Costantino U, Marmottini F, Nocchetti M, Vivani R (1998) New Synthetic routes to hydrotalcite-like compounds—characterisation and properties of the obtained materials. Eur J Inorg Chem 1998(10), 1439–1446. https://doi.org/10.1002/(SICI)1099-0682(199810)1998:10<1439::AID-EJIC1439>3.0.CO;2-1
Xu S et al (2015) Mg–Fe mixed oxides as solid base catalysts for the transesterification of microalgae oil. RSC Adv 5(87):71278–71286. https://doi.org/10.1039/C5RA14144C
Jitianu M, Zaharescu M, Bãlãsoiu M, Jitianu A (2003) The sol-gel route in synthesis of Cr(III)-containing clays. Comparison between Mg–Cr and Ni–Cr anionic clays. J Sol Gel Sci Technol 26(1):217–221. https://doi.org/10.1023/A:1020747031524
Mandal S, Tichit D, Lerner DA, Marcotte N (2009) Azoic dye hosted in layered double hydroxide: physicochemical characterization of the intercalated materials. Langmuir 25(18):10980–10986. https://doi.org/10.1021/la901201s
Liu J, Zhou Q, Chen J, Zhang L, Chang N (2013) Phosphate adsorption on hydroxyl–iron–lanthanum doped activated carbon fiber. Chem Eng J 215–216:859–867. https://doi.org/10.1016/j.cej.2012.11.067
Miyata S (1980) Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner 28(1):50–56. https://doi.org/10.1346/CCMN.1980.0280107
Raki L, Beaudoin JJ, Mitchell L (2004) Layered double hydroxide-like materials: nanocomposites for use in concrete. Cement Concrete Res 34(9):1717–1724. https://doi.org/10.1016/j.cemconres.2004.05.012
Xing K, Wang H, Guo L, Song W, Zhao Z (2008) Adsorption of tripolyphosphate from aqueous solution by Mg–Al–CO3-layered double hydroxides. Colloids Surf A Physicochem Eng Asp 328(1):15–20. https://doi.org/10.1016/j.colsurfa.2008.06.015
Yang K et al (2014) Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: kinetics, isotherms and mechanisms. Separ Purif Technol 124:36–42. https://doi.org/10.1016/j.seppur.2013.12.042
Adachi-Pagano M, Forano C, Besse J-P (2003) Synthesis of Al-rich hydrotalcite-like compounds by using the urea hydrolysis reaction—control of size and morphology. J Mater Chem 13(8):1988–1993. https://doi.org/10.1039/B302747N
Ogawa M, Kaiho H (2002) Homogeneous precipitation of uniform hydrotalcite particles. Langmuir 18(11):4240–4242. https://doi.org/10.1021/la0117045
Zeng H-Y, Deng X, Wang Y-J, Liao K-B (2009) Preparation of Mg–Al hydrotalcite by urea method and its catalytic activity for transesterification. AIChE J 55(5):1229–1235. https://doi.org/10.1002/aic.11722
Benhiti R, Ait Ichou A, Zaghloul A, Aziam R, Carja G, Zerbet M, Chiban M (2020) Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ Sci Pollut Res 27(36):45767–45774. https://doi.org/10.1007/s11356-020-10444-5
Shaw WHR, Bordeaux JJ (1955) The decomposition of urea in aqueous media. J Am Chem Soc 77(18):4729–4733. https://doi.org/10.1021/ja01623a011
Tian L, Ma W, Han M (2010) Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem Eng J 156(1):134–140. https://doi.org/10.1016/j.cej.2009.10.008
Rodier J, Legube B, Merlet N (2016) L’analyse de l’eau—10e édition; Eaux naturelles, eaux résiduaires, eau de mer. Technique et ingénierie, Dunod
Novillo C, Guaya D, Avendaño AA-P, Armijos C, Cortina JL, Cota I (2014) Evaluation of phosphate removal capacity of Mg/Al layered double hydroxides from aqueous solutions. Fuel 138:72–79. https://doi.org/10.1016/j.fuel.2014.07.010
Liu H, Sun X, Yin C, Hu C (2008) Removal of phosphate by mesoporous ZrO2. J Hazard Mater 151(2):616–622. https://doi.org/10.1016/j.jhazmat.2007.06.033
Wang Y, Zhang F, Xu S, Wang X, Evans DG, Duan X (2008) Preparation of layered double hydroxide microspheres by spray drying. Ind Eng Chem Res 47(15):5746–5750. https://doi.org/10.1021/ie800146m
Ookubo A (1992) Hydrotalcites as potential adsorbents of intestinal phophate. J Pharm Sci 81:1139–1140. https://doi.org/10.1002/jps.2600811120
Zhou J, Yang S, Yu J, Shu Z (2011) Novel hollow microspheres of hierarchical zinc–aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water. J Hazard Mater 192(3):1114–1121. https://doi.org/10.1016/j.jhazmat.2011.06.013
Zak AK, Majid WHA, Abrishami ME, Yousefi R (2011) X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci 13(1):251–256. https://doi.org/10.1016/j.solidstatesciences.2010.11.024
Goh K-H, Lim T-T, Dong Z (2008) Application of layered double hydroxides for removal of oxyanions: a review. Water Res 42(6):1343–1368. https://doi.org/10.1016/j.watres.2007.10.043
Khitous M, Salem Z, Halliche D (2016) Removal of phosphate from industrial wastewater using uncalcined MgAl–NO3 layered double hydroxide: batch study and modeling. Desal Water Treat 57(34):15920–15931. https://doi.org/10.1080/19443994.2015.1077745
Rodriguez LEG, Bail A, Castillo RO, Arízaga GGC (2020) Removal and extraction of carboxylic acids and non-ionic compounds with simple hydroxides and layered double hydroxides. Curr Pharm Des 26(6):650–663. https://doi.org/10.2174/1381612826666191226103623
Catts JG, Langmuir D (1986) Adsorption of Cu, Pb and Zn by δMnO2: applicability of the site binding-surface complexation model. Appl Geochem 1(2):255–264. https://doi.org/10.1016/0883-2927(86)90010-7
Honeyman BD, Santschi PH (1988) Metals in aquatic systems. Environ Sci Technol 22(8):862–871. https://doi.org/10.1021/es00173a002
Everaert M, Dox K, Steele JA, De Vos D, Smolders E (2019) Solid-state speciation of interlayer anions in layered double hydroxides. J Colloid Interface Sci 537:151–162. https://doi.org/10.1016/j.jcis.2018.11.010
Lundehøj L, Jensen HC, Wybrandt L, Nielsen UG, Christensen ML, Quist-Jensen CA (2019) Layered double hydroxides for phosphorus recovery from acidified and non-acidified dewatered sludge. Water Res 153:208–216. https://doi.org/10.1016/j.watres.2019.01.004
Li R et al (2016) Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci Total Environ 559:121–129. https://doi.org/10.1016/j.scitotenv.2016.03.151
Jung K-W, Lee S, Lee YJ (2017) Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour Technol 245:751–759. https://doi.org/10.1016/j.biortech.2017.09.035
Seida Y, Nakano Y (2002) Removal of phosphate by layered double hydroxides containing iron. Water Res 36(5):1306–1312. https://doi.org/10.1016/S0043-1354(01)00340-2
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Yogendrarajah P, Samapundo S, Devlieghere F, De Saeger S, De Meulenaer B (2015) Moisture sorption isotherms and thermodynamic properties of whole black peppercorns (Pipernigrum L.). LWT Food Sci Technol 64(1):177–188. https://doi.org/10.1016/j.lwt.2015.05.045
Yakar A, Ünlü A, Yeşilçayır T, Bıyık İ (2020) Kinetics and thermodynamics of textile dye removal by adsorption onto iron oxide nanoparticles. Nanotechnol Environ Eng 5(1):6. https://doi.org/10.1007/s41204-020-0068-0
Saha B, Das S, Saikia J, Das G (2011) Preferential and enhanced adsorption of different dyes on iron oxide nanoparticles: a comparative study. J Phys Chem C 115(16):8024–8033. https://doi.org/10.1021/jp109258f
Ivanets AI, Shashkova IL, Kitikova NV, Morozov Y (2016) The kinetic studies of the cobalt ion removal from aqueous solutions by dolomite-based sorbent. Int J Environ Sci Technol 13(11):2561–2568. https://doi.org/10.1007/s13762-016-1089-x
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/j.watres.2017.04.014
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10. https://doi.org/10.1016/j.cej.2009.09.013
Lekkas PT (2020) Thermally treated aluminium waste-filings, a low cost and efficient adsorbent for phosphorus removal from water, sept. 26, 2018. https://journal.gnest.org/publication/gnest_02562 (consulté le juill. 20, 2020)
Vasudevan S, Lakshmi J (2012) The adsorption of phosphate by graphene from aqueous solution. RSC Adv 2(12):5234–5242. https://doi.org/10.1039/C2RA20270K
Yu S-H, Dong X-L, Gong H, Jiang H, Liu Z-G (2012) Adsorption kinetic and thermodynamic studies of phosphate onto tantalum hydroxide. Water Environ Res 84(12):2115–2122. https://doi.org/10.2175/106143012X13415215906933
Zhang J et al (2010) Adsorption behavior of phosphate on Lanthanum(III) doped mesoporous silicates material. J Environ Sci 22(4):507–511. https://doi.org/10.1016/S1001-0742(09)60141-8
Tran HN, You S-J, Chao H-P (2016) Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J Environ Chem Eng 4(3):2671–2682. https://doi.org/10.1016/j.jece.2016.05.009
Hibino T (2018) Anion selectivity of layered double hydroxides: effects of crystallinity and charge density. Eur J Inorgan Chem 2018(6):722–730. https://doi.org/10.1002/ejic.201701067
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s41204-023-00315-y
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benhiti, R., Ait Ichou, A., Zaghloul, A. et al. RETRACTED ARTICLE: Kinetic, isotherm, thermodynamic and mechanism investigations of dihydrogen phosphate removal by MgAl-LDH. Nanotechnol. Environ. Eng. 6, 16 (2021). https://doi.org/10.1007/s41204-021-00110-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-021-00110-7