Abstract
Ligament, cartilage, and meniscus injuries often have poor healing due to low vascularity and low proliferative abilities of the resident cells. Drawbacks with conventional treatment methodologies have prompted interest in a new approach we term “Regenerative Engineering” to regenerate orthopaedic tissues. The work of cells is of central importance in the Regenerative Engineering paradigm. In this regard, both differentiated cells and stem cells such as bone marrow stromal cells have been studied as sources for orthopaedic tissue regeneration. In addition, other stem cells such as those derived from peripheral blood, synovium, adipose, and other extraembryonic sources have been isolated and characterized and subsequently investigated for regenerating various orthopaedic tissues. In this review, recent developments in the stem cell-mediated regeneration of ligament, cartilage, and menisci are discussed.
Lay Summary
Most orthopaedic tissue ailments originate from trauma or degenerative diseases. Commonly utilized strategies in clinical settings have shortcomings such as poor or incomplete healing. By converging advanced materials science with stem cells, growth factors/small molecules, and developmental biology, regenerative engineering is expected to provide strategies for orthopaedic tissue regeneration. In this review, we discuss various cell sources that have been isolated, characterized, and studied for regenerating orthopaedic tissues. Some of the underlying molecular mechanisms involved in those cells are also discussed. In addition, various approaches based on those cell sources for regenerating ligament, cartilage, and meniscus tissues are reported. In the future, cell-based approaches discussed in this review need to be combined with other salient aspects of regenerative engineering to facilitate activation of multiple signaling pathways required for tissue regeneration. Via such a holistic approach, we anticipate regeneration of ligaments, cartilage, and meniscus with features similar to that of native tissue.






Similar content being viewed by others
References
United States Bone and Joint Initiative: the burden of musculoskeletal diseases in the United States (BMUS). Third edition, Rosemont, IL 2014, http://www.boneandjointburden.org/facts-brief. 2016.
Mather RC, Hettrich CM, Dunn WR, Cole BJ, Bach BR Jr, Huston LJ, et al. Cost-effectiveness analysis of early reconstruction versus rehabilitation and delayed reconstruction for anterior cruciate ligament tears. Am J Sports Med. 2014;42:1583–91.
Maffulli N, Longo UG, Campi S, Denaro V. Meniscal tears. Open Access Journal of Sports Medicine. 2010;1:45–54.
Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19.
Stringham DR, Pelmas CJ, Burks RT, Newman AP, Marcus RL. Comparison of anterior cruciate ligament reconstructions using patellar tendon autograft or allograft. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1996;12:414–21.
Gorschewsky O, Klakow A, Pütz A, Mahn H, Neumann W. Clinical comparison of the autologous quadriceps tendon (BQT) and the autologous patella tendon (BPTB) for the reconstruction of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15:1284–92.
Eriksson K, Anderberg P, Hamberg P, Olerud P, Wredmark T. There are differences in early morbidity after ACL reconstruction when comparing patellar tendon and semitendinosus tendon graft. Scand J Med Sci Sports. 2001;11:170–7.
Ejerhed L, Kartus J, Sernert N, Köhler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? Am J Sports Med. 2003;31:19–25.
Sun K, Tian S-q, Zhang J-h, Xia C-s, Zhang C-l, Yu T-b. ACL reconstruction with BPTB autograft and irradiated fresh frozen allograft. Journal of Zhejiang University SCIENCE B 2009;10:306–316.
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. The Journal of Bone & Joint Surgery. 2004;86:455.
Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee. Am J Sports Med. 2009;37:42–55.
Tohyama H, Yasuda K, Minami A, Majima T, Iwasaki N, Muneta T, et al. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14:579–88.
D’Anchise R, Manta N, Prospero E, Bevilacqua C, Gigante A. Autologous implantation of chondrocytes on a solid collagen scaffold: clinical and histological outcomes after two years of follow-up. Journal of Orthopaedics andTraumatology. 2005;6:36–43.
Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold. Am J Sports Med. 2014;42:1618–27.
Kreuz PC, Müller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Research & Therapy. 2009;11:R33.
Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage. Am J Sports Med. 2014;42:1417–25.
Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Review of Medical Devices. 2006;3:49–57.
Kwansa AL, Empson YM, Ekwueme EC, Walters VI, Freeman JW, Laurencin CT. Novel matrix based anterior cruciate ligament (ACL) regeneration. Soft Matter. 2010;6:5016–25.
Fishman JA, Greenwald MA. Grossi PA. Essential Considerations in Donor Screening. Clinical Infectious Diseases: Transmission of infection with human allografts; 2012.
Galili U, LaTemple DC, Walgenbach AW, Stone KR. Porcine and bovine cartilage transplants in cynomolgus monkey. Transplantation. 1997;63:646–51.
Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–9.
Stone KR, Walgenbach AW, Turek TJ, Somers DL, Wicomb W, Galili U. Anterior cruciate ligament reconstruction with a porcine xenograft: a serologic, histologic, and biomechanical study in primates. Arthroscopy. 2007;23:411-9.e1.
Amini AR, Wallace JS, Nukavarapu SP. Short-term and long-term effects of orthopedic biodegradable implants. J Long-Term Eff Med Implants. 2011;21:93–122.
Yu X, Tang X, Gohil SV, Laurencin CT. Biomaterials for bone regenerative engineering. Advanced Healthcare Materials. 2015;4:1268–85.
Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. 2011;49:832–64.
Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016;107:247–76.
Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015;46:1–24.
Narayanan G, Gupta BS, Tonelli AE. Poly(ε-caprolactone) nanowebs functionalized with α- and γ-cyclodextrins. Biomacromolecules. 2014;15:4122–33.
Narayanan G, Gupta BS, Tonelli AE. Enhanced mechanical properties of poly (ε-caprolactone) nanofibers produced by the addition of non-stoichiometric inclusion complexes of poly (ε-caprolactone) and α-cyclodextrin. Polymer. 2015;76:321–30.
Narayanan G, Aguda R, Hartman M, Chung C-C, Boy R, Gupta BS, et al. Fabrication and characterization of poly(ε-caprolactone)/α-cyclodextrin pseudorotaxane nanofibers. Biomacromolecules. 2016;17:271–9.
Narayanan G, Ormond BR, Gupta BS, Tonelli AE. Efficient wound odor removal by β-cyclodextrin functionalized poly (ε-caprolactone) nanofibers. Journal of Applied Polymer Science. 2015.;132:DOI: 10.1002/app.42782.
Narayanan G, Chung C-C, Aguda R, Boy R, Hartman M, Mehraban N, et al. Correlation of the stoichiometries of poly(ε-caprolactone) and [small alpha]-cyclodextrin pseudorotaxanes with their solution rheology and the molecular orientation, crystallite size, and thermomechanical properties of their nanofibers. RSC Adv. 2016;6:111326–36.
Bhattacharjee M, Chameettachal S, Pahwa S, Ray AR, Ghosh S. Strategies for replicating anatomical cartilaginous tissue gradient in engineered intervertebral disc. ACS Appl Mater Interfaces. 2014;6:183–93.
Bhattacharjee M, Miot S, Gorecka A, Singha K, Loparic M, Dickinson S, et al. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: towards annulus fibrosus tissue engineering. Acta Biomater. 2012;8:3313–25.
Bhattacharjee M, Schultz-Thater E, Trella E, Miot S, Das S, Loparic M, et al. The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials. Biomaterials. 2013;34:8161–71.
Narayanan G, Tekbudak MY, Caydamli Y, Dong J, Krause WE. Accuracy of electrospun fiber diameters: the importance of sampling and person-to-person variation. Polym Test. 2017;61:240–8.
Lo KWH, Ulery BD, Deng M, Ashe KM, Laurencin CT. Current patents on osteoinductive molecules for bone tissue engineering. Recent Pat Biomed Eng. 2011;4:153–67.
Lo KWH, Kan HM, Ashe KM, Laurencin CT. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J Tissue Eng Regen Med. 2012;6:40–8.
Lo KWH, Ashe KM, Kan HM, Laurencin CT. The role of small molecules in musculoskeletal regeneration. Regen Med. 2012;7:535–49.
Lo KWH, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64:1277–91.
Ficklscherer A, Serr M, Loitsch T, Niethammer TR, Lahner M, Pietschmann MF, et al. The influence of different footprint preparation techniques on tissue regeneration in rotator cuff repair in an animal model. Arch Med Sci. 2016;13:481–8.
Lo KWH, Kan HM, Gagnon KA, Laurencin CT. One-day treatment of small molecule 8-bromo-cyclic AMP analogue induces cell-based VEGF production for in vitro angiogenesis and osteoblastic differentiation. J Tissue Eng Regen Med. 2016;10:867–75.
Lo KWH, Jiang T, Gagnon KA, Nelson C, Laurencin CT. Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 2014;32:74–81.
Cushnie EK, Ulery BD, Nelson SJ, Deng M, Sethuraman S, Doty SB, et al. Simple signaling molecules for inductive bone regenerative engineering. PLoS One. 2014;9:e101627.
Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KWH. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov Today. 2014;19:794–800.
Lo KWH, Ulery BD, Kan HM, Ashe KM, Laurencin CT. Evaluating the feasibility of utilizing the small molecule phenamil as a novel biofactor for bone regenerative engineering. J Tissue Eng Regen Med. 2014;8:728–36.
Carbone EJ, Rajpura K, Jiang T, Laurencin CT, Lo KW-H. Regulation of bone regeneration with approved small molecule compounds 2014.
Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–83.
Laurencin CT, Nair LS. Regenerative engineering: approaches to limb regeneration and other grand challenges. Regenerative Engineering and Translational Medicine. 2015;1:1–3.
Ingber DE, Levin M. What lies at the interface of regenerative medicine and developmental biology? Development. 2007;134:2541–7.
McCusker C, Lehrberg J, Gardiner D. Position-specific induction of ectopic limbs in non-regenerating blastemas on axolotl forelimbs. Regeneration. 2014;1:27–34.
McCusker C, Bryant SV, Gardiner DM. The axolotl limb blastema: cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration. 2015;2:54–71.
Lehrberg J, Gardiner DM. Regulation of axolotl (Ambystoma mexicanum) limb blastema cell proliferation by nerves and BMP2 in organotypic slice culture. PLoS One. 2015;10:e0123186.
Laurencin CT, Nair LS. The quest toward limb regeneration: a regenerative engineering approach. Regenerative Biomaterials. 2016;3:123–5.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408.
Bahney C, Miclau T. Therapeutic potential of stem cells in orthopedics. Indian Journal of Orthopaedics. 2012;46:4–9.
Kornblum HI. Introduction to neural stem cells. Stroke. 2007;38:810–6.
Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49.
Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21.
Alison MR. Liver stem cells. Stem Cell Rev. 2005;1:253–60.
Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci. 2000;97:14720–5.
Amini AR, Laurencin CT, Nukavarapu SP. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res. 2012;30:1507–15.
Mangano C, Paino F, d'Aquino R, De Rosa A, Iezzi G, Piattelli A, et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocomplex. PLoS One. 2011;6:e18721.
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, FHJ C, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9.
Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–62.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Gertow K, Cedervall J, Unger C, Szöke K, Blennow E, Imreh MP, et al. Trisomy 12 in HESC leads to no selective in vivo growth advantage in teratomas, but induces an increased abundance of renal development. J Cell Biochem. 2007;100:1518–25.
Wakitani S, Takaoka K, Hattori T, Miyazawa N, Iwanaga T, Takeda S, et al. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology. 2003;42:162–5.
Eisenstein M. IPSCs: one cell to rule them all? Nat Meth. 2010;7:81–5.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55.
Guzzo RM, Scanlon V, Sanjay A, Xu R-H, Drissi H. Establishment of human cell type-specific iPS cells with enhanced chondrogenic potential. Stem Cell Rev Rep. 2014;10:820–9.
Lietman SA. Induced pluripotent stem cells in cartilage repair. World Journal of Orthopedics. 2016;7:149–55.
Bilic J, Belmonte JCI. Concise review: induced pluripotent stem cells versus embryonic stem cells: close enough or yet too far apart? Stem Cells. 2012;30:33–41.
Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Mácia A, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–70.
Baker M. Why hES cells make teratomas. 2009.
Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198–210.
Herbort M, Tecklenburg K, Zantop T, Raschke MJ, Hoser C, Schulze M, et al. Single-bundle anterior cruciate ligament reconstruction: a biomechanical cadaveric study of a rectangular quadriceps and bone–patellar tendon–bone graft configuration versus a round hamstring graft. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2013;29:1981–90.
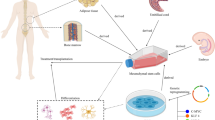
Caplan AI. Are all adult stem cells the same? Regenerative Engineering and Translational Medicine. 2015;1:4–10.
Beane OS, Darling EM. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann Biomed Eng. 2012;40:2079–97.
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. Journal of Embryology and Experimental Morphology. 1966;16:381–90.
Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–211.
van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartil. 2012;20:1186–96.
Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–25.
Schmitt A, van Griensven M, Imhoff AB, Buchmann S. Application of stem cells in orthopedics. Stem Cells Int. 2012;2012:11.
Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–70.
Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685.
Kasir R, Vernekar VN, Laurencin CT. Regenerative engineering of cartilage using adipose-derived stem cells. Regenerative Engineering and Translational Medicine. 2015;1:42–9.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4.
Zheng Y-H, Xiong WEI, Su KAI, Kuang S-J, Zhang Z-G. Multilineage differentiation of human bone marrow mesenchymal stem cells in vitro and in vivo. Experimental and Therapeutic Medicine. 2013;5:1576–80.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 15:641–8.
Lv F-J, Tuan RS, Cheung KMC, Leung VYL. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19.
Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–64.
Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84.
Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408.
Reinhardt M, Bader A, Giri S. Devices for stem cell isolation and delivery: current need for drug discovery and cell therapy. Expert Review of Medical Devices. 2015;12:353–64.
Körbling M, Burke P, Braine H, Elfenbein G, Santos G, Kaizer H. Successful engraftment of blood derived normal hemopoietic stem cells in chronic myelogenous leukemia. Exp Hematol. 1981;9:684–90.
Bensinger WI, Clift RA, Anasetti C, Appelbaum FA, Demirer T, Rowley S, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony stimulating factor. Stem Cells. 1996;14:90–105.
Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World Journal of Stem Cells. 2015;7:470–6.
Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40.
Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126–31.
Fernandez M, Simon V, Herrera G, Cao C, Del Favero H, Minguell J. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997;20
Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Research & Therapy. 2000;2:477.
Rodriguez-Fontan F, Piuzzi NS, Chahla J, Payne KA, LaPrade RF, Muschler GF, et al. Stem and progenitor cells for cartilage repair: source, safety, evidence, and efficacy, operative techniques in sports medicine. 2017;25:25–33.
Hopper N, Wardale J, Brooks R, Power J, Rushton N, Henson F. Peripheral blood mononuclear cells enhance cartilage repair in in vivo osteochondral defect model. PLoS One. 2015;10:e0133937.
Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–12.
Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45.
Kuznetsov SA, Mankani MH, Leet AI, Ziran N, Gronthos S, Robey PG. Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells. 2007;25:1830–9.
Cesselli D, Beltrami AP, Rigo S, Bergamin N, D'Aurizio F, Verardo R, et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res. 2009;104:1225–34.
Civriz Bozdag S, Bay M, Ayyıldız E, Topcuoglu P, Ilhan O. Older age and capacity of colony forming unit in autologous peripheral derived hematopoietic cells. Transfus Apher Sci. 2012;47:113–6.
de Sousa EB, Casado PL, Neto VM, Duarte MEL, Aguiar DP. Synovial fluid and synovial membrane mesenchymal stem cells: latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014;5:112.
Iwanaga T, Shikichi M, Kitamura H, Yanase H, Nozawa-Inoue K. Morphology and functional roles of synoviocytes in the joint. Arch Histol Cytol. 2000;63:17–31.
Blom AB, van Lent PLEM, Holthuysen AEM, van der Kraan PM, Roth J, van Rooijen N, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2004;12:627–35.
Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, et al. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis & Rheumatism. 2008;58:1731–40.
Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57.
Matsukura Y, Muneta T, Tsuji K, Koga H, Sekiya I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin Orthop Relat Res. 2014;472:1357–64.
Lee DH, Sonn CH, Han SB, Oh Y, Lee KM, Lee SH. Synovial fluid CD34− CD44+ CD90+ mesenchymal stem cell levels are associated with the severity of primary knee osteoarthritis. Osteoarthr Cartil. 2012;20:106–9.
Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis & Rheumatism. 2004;50:817–27.
Hoff P, Buttgereit F, Burmester G-R, Jakstadt M, Gaber T, Andreas K, et al. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37:145–51.
Zoltan S, Gabriella S, Sandor S, Alisa EK. Chemokines in rheumatic diseases. Curr Drug Targets. 2006;7:91–102.
Röhner E, Matziolis G, Perka C, Füchtmeier B, Gaber T, Burmester G-R, et al. Inflammatory synovial fluid microenvironment drives primary human chondrocytes to actively take part in inflammatory joint diseases. Immunol Res. 2012;52:169–75.
Smith MD, Barg E, Weedon H, Papengelis V, Smeets T, Tak PP, et al. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann Rheum Dis. 2003;62:303–7.
De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis & Rheumatism. 2001;44:1928–42.
Harvanová D, Tóthová T, Sarisský M, Amrichová J, Rosocha J. Isolation and characterization of synovial mesenchymal stem cells. Folia Biol. 2011;57:119.
HERMIDA-GÓMEZ T, FUENTES-BOQUETE I, GIMENO-LONGAS MJ, MUIÑOS-LÓPEZ E, DÍAZ-PRADO S, de TORO FJ, et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol. 2011;38:339–49.
Arufe MC, De la Fuente A, Fuentes-Boquete I, De Toro FJ, Blanco FJ. Differentiation of synovial CD-105+ human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145–55.
Arufe MC, De la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111:834–45.
Krawetz RJ, Wu YE, Martin L, Rattner JB, Matyas JR, Hart DA. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One. 2012;7:e43616.
Mak J, Jablonski CL, Leonard CA, Dunn JF, Raharjo E, Matyas JR, et al. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci Rep. 2016;6:23076.
Zheng Y-L, Sun Y-P, Zhang H, Liu W-J, Jiang R, Li W-Y, et al. Mesenchymal stem cells obtained from synovial fluid mesenchymal stem cell-derived induced pluripotent stem cells on a Matrigel coating exhibited enhanced proliferation and differentiation potential. PLoS One. 2015;10:e0144226.
Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis & Rheumatism. 2006;54:3075–8.
Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng B Rev. 2012;18:301–11.
Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol. 2012;227:2163–74.
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis & Rheumatism. 2005;52:2521–9.
Garcia J, Wright K, Roberts S, Kuiper JH, Mangham C, Richardson J, et al. Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: the influence of tissue source and inflammatory stimulus. Sci Rep. 2016;6:24295.
Sabapathy V, Sundaram B, Vm S, Mankuzhy P, Kumar S. Human Wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9:e93726.
Kim D-W, Staples M, Shinozuka K, Pantcheva P, Kang S-D, Borlongan C. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692.
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267–82.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Troyer DL, Weiss ML. Concise review: Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–9.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42.
Lee OK, Kuo TK, Chen W-M, Lee K-D, Hsieh S-L, Chen T-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–75.
McElreavey KD, Irvine AI, Ennis KT, McLean WHI. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton's jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19:29S.
Wang H-S, Hung S-C, Peng S-T, Huang C-C, Wei H-M, Guo Y-J, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7.
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotech. 2007;25:100–6.
Chen X, Zhang F, He X, Xu Y, Yang Z, Chen L, et al. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury. 2013;44:540–9.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Davydova DA, Vorotelyak EA, Smirnova Y, Zinovieva RD, Romanov Y, Kabaeva NV, et al. Cell phenotypes in human amniotic fluid. Acta Nat. 2009;1:98–103.
Aicher WK, Bühring H-J, Hart M, Rolauffs B, Badke A, Klein G. Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells—potential and pitfalls. Adv Drug Deliv Rev. 2011;63:342–51.
Rozemuller H, Prins H-J, Naaijkens B, Staal J, Bühring H-J, Martens AC. Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cells Dev. 2010;19:1911–21.
Farias VA, Linares-Fernández JL, Peñalver JL, Payá Colmenero JA, Ferrón GO, Duran EL, et al. Human umbilical cord stromal stem cell express CD10 and exert contractile properties. Placenta. 2011;32:86–95.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second- trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–6.
Arnhold S, Gluer S, Hartmann K, Raabe O, Addicks K, Wenisch S, et al. Amniotic-fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int. 2011;2011
Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4:423–33.
Oliveira MS, Barreto-Filho JB. Placental-derived stem cells: culture, differentiation and challenges. World Journal of Stem Cells. 2015;7:769–75.
Pelekanos RA, Li J, Gongora M, Chandrakanthan V, Scown J, Suhaimi N, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73.
Nazarov I, Lee JW, Soupene E, Etemad S, Knapik D, Green W, et al. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Transl Med. 2012;1:359–72.
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–92.
Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–5.
Talwadekar MD, Kale VP, Limaye LS. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts—a paired sample study. Sci Rep. 2015;5:15784.
Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8.
Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ, Oh W, et al. Downregulation of melanoma cell adhesion molecule (MCAM/CD146) accelerates cellular senescence in human umbilical cord blood-derived mesenchymal stem cells. Stem Cells Transl Med. 2016;5:427–39.
Nekanti U, Mohanty L, Venugopal P, Balasubramanian S, Totey S, Ta M. Optimization and scale-up of Wharton's jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5:244–54.
Chitteti BR, Kobayashi M, Cheng Y, Zhang H, Poteat BA, Broxmeyer HE, et al. CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood. 2014;124:519–29.
Noort WA, Oerlemans MIFJ, Rozemuller H, Feyen D, Jaksani S, Stecher D, et al. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. 2012;16:1827–39.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod (Oxf, Engl). 2004;19:1450–6.
Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang P-H, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–35.
Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2006;22:441–51.
Lewis SL, Bucher L, Heitkemper MM, Harding MM, Kwong J, Roberts D. Medical-surgical nursing: assessment and management of clinical problems, Single volume. St. Louis: Elsevier Health Sciences; 2016.
Freeman JW, Woods MD, Laurencin CT. Tissue engineering of the anterior cruciate ligament using a braid–twist scaffold design. J Biomech. 2007;40:2029–36.
Ferretti M, Levicoff EA, Macpherson TA, Moreland MS, Cohen M, Fu FH. The fetal anterior cruciate ligament: an anatomic and histologic study. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2007;23:278–83.
Leong NL, Petrigliano FA, McAllister DR. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J Biomed Mater Res A. 2014;102:1614–24.
Frank C, Woo SL-Y, Amiel D, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing. Am J Sports Med. 1983;11:379–89.
Keene GCR, Bickerstaff D, Rae PJ, Paterson RS. The natural history of meniscal tears in anterior cruciate ligament insufficiency. Am J Sports Med. 1993;21:672–9.
Doroski DM, Brink KS, Temenoff JS. Techniques for biological characterization of tissue-engineered tendon and ligament. Biomaterials. 2007;28:187–202.
Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983;172:19–25.
Laurencin CT, Ambrosio A, Borden M, Cooper J Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46.
Petersen W, Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35–47.
Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363–71.
Bellincampi LD, Closkey RF, Prasad R, Zawadsky JP, Dunn MG. Viability of fibroblast-seeded ligament analogs after autogenous implantation. J Orthop Res. 1998;16:414–20.
Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–32.
Lu HH, Cooper JA Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16.
Laurencin CT, Freeman JW. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530–6.
Freeman JW, Woods MD, Cromer DA, Ekwueme EC, Andric T, Atiemo EA, et al. Evaluation of a hydrogel–fiber composite for ACL tissue engineering. J Biomech. 2011;44:694–9.
Li W-J, Cooper JA Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–85.
Meaney Murray M, Spector M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. 2001;22:2393–402.
Majima T, Funakosi T, Iwasaki N, Yamane S-T, Harada K, Nonaka S, et al. Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J Orthop Sci. 2005;10:302–7.
Shao H-J, Chen CS, Lee Y-T, Wang J-H, Young T-H. The phenotypic responses of human anterior cruciate ligament cells cultured on poly(ϵ-caprolactone) and chitosan. J Biomed Mater Res A. 2010;93A:1297–305.
Majima T, Irie T, Sawaguchi N, Funakoshi T, Iwasaki N, Harada K, et al. Chitosan-based hyaluronan hybrid polymer fibre scaffold for ligament and tendon tissue engineering. Proc Inst Mech Eng H J Eng Med. 2007;221:537–46.
Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–41.
Nau T, Teuschl A. Regeneration of the anterior cruciate ligament: current strategies in tissue engineering. World J Orthop. 2015;6:127–36.
Liu W, Chen B, Deng D, Xu F, Cui L, Cao Y. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng. 2006;12:775–8.
Cooper JA Jr, Bailey LO, Carter JN, Castiglioni CE, Kofron MD, Ko FK, et al. Evaluation of the anterior cruciate ligament, medial collateral ligament, Achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27:2747–54.
Kato S, Saito M, Funasaki H, Marumo K. Distinctive collagen maturation process in fibroblasts derived from rabbit anterior cruciate ligament, medial collateral ligament, and patellar tendon in vitro. Knee Surg Sports Traumatol Arthrosc. 2015;23:1384–92.
Ge Z, Goh JCH, Lee EH. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573–83.
Cooper JA, Sahota JS, Gorum WJ, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci. 2007;104:3049–54.
Fan H, Liu H, Toh SL, Goh JCH. Enhanced differentiation of mesenchymal stem cells co-cultured with ligament fibroblasts on gelatin/silk fibroin hybrid scaffold. Biomaterials. 2008;29:1017–27.
Fan H, Liu H, Toh SL, Goh JCH. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967–77.
Mifune Y, Matsumoto T, Takayama K, Terada S, Sekiya N, Kuroda R, et al. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials. 2013;34:5476–87.
Liu H, Wei X, Ding X, Li X, Zhou G, Li P, et al. Comparison of cellular responses of mesenchymal stem cells derived from bone marrow and synovium on combined silk scaffolds. J Biomed Mater Res A. 2015;103:115–25.
Zhang N, Dietrich MA, Lopez MJ. Canine intra-articular multipotent stromal cells (MSC) from adipose tissue have the highest in vitro expansion rates, multipotentiality, and MSC immunophenotypes. Vet Surg. 2013;42:137–46.
Proffen BL, Haslauer CM, Harris CE, Murray MM. Mesenchymal stem cells from the retropatellar fat pad and peripheral blood stimulate ACL fibroblast migration, proliferation, and collagen gene expression. Connect Tissue Res. 2013;54:14–21.
Ouyang HW, Goh JCH, Mo XM, Teoh SH, Lee EH. Characterization of anterior cruciate ligament cells and bone marrow stromal cells on various biodegradable polymeric films. Mater Sci Eng C. 2002;20:63–9.
Liu H, Fan H, Toh SL, Goh JCH. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443–53.
Sahoo S, Ouyang H, Goh JC-H, Tay T, Toh S. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91–9.
Gantenbein B, Gadhari N, Chan SCW, Kohl S, Ahmad SS. Mesenchymal stem cells and collagen patches for anterior cruciate ligament repair. World Journal of Stem Cells. 2015;7:521–34.
Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, Mcallister DR. The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue Eng. 2007;13:2721–31.
Sahoo S, Toh SL, Goh JCH. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–8.
Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–53.
Liu A, Xue G-H, Sun M, Shao H-F, Ma C-Y, Gao Q, et al. 3D printing surgical implants at the clinic: a experimental study on anterior cruciate ligament reconstruction. Sci Rep. 2016;6:21704.
Yilgor C, Yilgor Huri P, Huri G. Tissue engineering strategies in ligament regeneration. Stem Cells Int. 2012;2012:9.
Nau T, Teuschl A. Regeneration of the anterior cruciate ligament: current strategies in tissue engineering. World Journal of Orthopedics. 2015;6:127–36.
Hoffmann A, Gross G. Tendon and ligament engineering: from cell biology to in vivo application. Regen Med. 2006;1:563–74.
Dong Y, Zhang Q, Li Y, Jiang J, Chen S. Enhancement of tendon–bone healing for anterior cruciate ligament (ACL) reconstruction using bone marrow-derived mesenchymal stem cells infected with BMP-2. Int J Mol Sci. 2012;13:13605.
Wang C-J, Weng L-H, Hsu S-L, Sun Y-C, Yang Y-J, Chan Y-S, et al. pCMV–BMP-2-transfected cell-mediated gene therapy in anterior cruciate ligament reconstruction in rabbits. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2010;26:968–76.
Huang TF, Chen YT, Yang TH, Chen LL, Chiou SH, Tsai TH, et al. Isolation and characterization of mesenchymal stromal cells from human anterior cruciate ligament. Cytotherapy. 2008;10:806–14.
Cheng M-T, Liu C-L, Chen T-H, Lee OK. Comparison of potentials between stem cells isolated from human anterior cruciate ligament and bone marrow for ligament tissue engineering. Tissue Eng A. 2010;16:2237–53.
Sarah B, Bizunesh MB, Marieke Z, Tom M, Bert S, Marc S, et al. Intravenous application of allogenic peripheral blood-derived mesenchymal stem cells: a safety assessment in 291 equine recipients. Current Stem Cell Research & Therapy. 2014;9:452–7.
Vandenberghe A, Broeckx SY, Beerts C, Seys B, Zimmerman M, Verweire I, et al. Tenogenically induced allogeneic mesenchymal stem cells for the treatment of proximal suspensory ligament desmitis in a horse. Frontiers in Veterinary Science. 2015;2
Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26:819–30.
Uefuji A, Matsumoto T, Matsushita T, Ueha T, Zhang S, Kurosaka M, et al. Age-related differences in anterior cruciate ligament remnant vascular-derived cells. Am J Sports Med. 2014;42:1478–86.
Proffen BL, Vavken P, Haslauer CM, Fleming BC, Harris CE, Machan JT, et al. Addition of autologous mesenchymal stem cells to whole blood for bioenhanced ACL repair has no benefit in the porcine model. Am J Sports Med. 2015;43:320–30.
Buckwalter J, Mankin H. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1997;47:477–86.
Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure. Function, and Importance in Tissue Engineering. 2007;35:363–411.
Bhattacharjee M, Coburn J, Centola M, Murab S, Barbero A, Kaplan DL, et al. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–22.
Escobar JL, Ivirico MB, Kuyinu E, Nair LS, Laurencin CT. Regenerative engineering for knee osteoarthritis treatment: biomaterials and cell-based technologies. Engineering. 2017;3:16–27.
Fox AJS, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–8.
Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng B Rev. 2013;19:31–40.
Minas T, Gomoll AH, Solhpour S, Rosenberger R, Probst C, Bryant T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468:147–57.
Ossendorf C, Steinwachs MR, Kreuz PC, Osterhoff G, Lahm A, Ducommun PP, et al. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. Sports Med, Arthrosc, Rehabil, Ther Technol. 2011;3:11.
Viste A, Piperno M, Desmarchelier R, Grosclaude S, Moyen B, Fessy MH. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop Traumatol Surg Res. 2012;98:737–43.
Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24.
Barbero A, Grogan SP, Mainil-Varlet P, Martin I. Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J Cell Biochem. 2006;98:1140–9.
Jenniskens YM, Koevoet W, de Bart AC, Weinans H, Jahr H, Verhaar JA, et al. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:1136–46.
Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, et al. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis & Rheumatism. 2008;58:197–208.
Scotti C, Osmokrovic A, Wolf F, Miot S, Peretti GM, Barbero A, et al. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1β and low oxygen. Tissue Eng A. 2011;18:362–72.
Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 388:1985–94.
Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246.
Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis & Rheumatism. 2012;64:3626–37.
Cucchiarini M, Venkatesan JK, Ekici M, Schmitt G, Madry H. Human mesenchymal stem cells overexpressing therapeutic genes: from basic science to clinical applications for articular cartilage repair. Biomed Mater Eng. 2012;22:197–208.
Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 2007;4:415–28.
Al Faqeh H, Nor Hamdan BMY, Chen HC, Aminuddin BS, Ruszymah BHI. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47:458–64.
Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Engineering Part C: Methods. 2009;15:647–58.
Li Q, Tang J, Wang R, Bei C, Xin L, Zeng Y, et al. Comparing the chondrogenic potential in vivo of autogeneic mesenchymal stem cells derived from different tissues. Artificial Cells, Blood Substitutes, and Biotechnology. 2011;39:31–8.
McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2011;27:1552–61.
Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–18.
Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647–54.
Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng A. 2009;16:523–33.
Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis & Rheumatism. 2006;54:1222–32.
Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–9.
Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G, et al. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30:2683–93.
Koh Y-G, Choi Y-J, Kwon S-K, Kim Y-S, Yeo J-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013;23:1308–16.
Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, et al. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Research & Therapy. 2013;15:1–16.
Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189–94.
Jurgens WJFM, Kroeze RJ, Zandieh-Doulabi B, van Dijk A, Renders GAP, Smit TH, et al. One-step surgical procedure for the treatment of osteochondral defects with adipose-derived stem cells in a caprine knee defect: a pilot study. BioResearch Open Access. 2013;2:315–25.
Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–66.
Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju Y-J, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–41.
Fan J, Varshney RR, Ren L, Cai D, Wang D-A. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng B Rev. 2009;15:75–86.
Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84–97.
Lee J-C, Lee SY, Min HJ, Han SA, Jang J, Lee S, et al. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng A. 2012;18:2173–86.
Lee J-C, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane–derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2013;29:1034–46.
MEASE PJ, HANNA S, FRAKES EP, ALTMAN RD. Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment. J Rheumatol. 2011;38:1546–51.
Shimomura K, Ando W, Tateishi K, Nansai R, Fujie H, Hart DA, et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31:8004–11.
Saw K-Y, Anz A, Merican S, Tay Y-G, Ragavanaidu K, Jee CSY, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2011;27:493–506.
Turajane T, Chaweewannakorn U, Larbpaiboonpong V, Aojanepong J, Thitiset T, Honsawek S, et al. Combination of intra-articular autologous activated peripheral blood stem cells with growth factor addition/preservation and hyaluronic acid in conjunction with arthroscopic microdrilling mesenchymal cell stimulation Improves quality of life and regenerates articular cartilage in early osteoarthritic knee disease. J Med Assoc Thail = Chotmaihet thangphaet. 2013;96:580–8.
Fu W-L, Ao Y-F, Ke X-Y, Zheng Z-Z, Gong X, Jiang D, et al. Repair of large full-thickness cartilage defect by activating endogenous peripheral blood stem cells and autologous periosteum flap transplantation combined with patellofemoral realignment. Knee. 2014;21:609–12.
Ossendorf C, Steinwachs MR, Kreuz PC, Osterhoff G, Lahm A, Ducommun PP, et al. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. Sports Med, Arthrosc, Rehabil, Ther Technol: SMARTT. 2011;3:11.
Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue engineering Part C, Methods. 2009;15:647–58.
Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23:647–54.
Koh Y-G, Choi Y-J, Kwon S-K, Kim Y-S, Yeo J-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23:1308–16.
Lee JC, Lee SY, Min HJ, Han SA, Jang J, Lee S, et al. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng Part A. 2012;18:2173–86.
Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193:161–78.
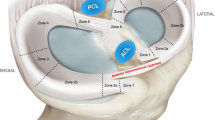
Maher SA, Rodeo SA, Warren RF. The meniscus. J Am Acad Orthop Surg. 2017;25:e18–e9.
Vaquero J, Forriol F. Meniscus tear surgery and meniscus replacement. Muscles, Ligaments and Tendons Journal. 2016;6:71–89.
Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. JBJS. 1983;65:538–47.
Fox AJS, Bedi A, Rodeo SA. The basic science of human knee menisci. Sports Health. 2011;4:340–51.
Pereira H, Silva-Correia J, Oliveira JM, Reis RL, Espregueira-Mendes J. The meniscus: basic science. In: Verdonk R, Espregueira Mendes J, Monllau JC, editors. Meniscal transplantation. Berlin: Springer; 2013. p. 7–14.
Kohn D, Moreno B. Meniscus insertion anatomy as a basis for meniscus replacement: a morphological cadaveric study. Arthroscopy. 1995;11:96–103.
Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31.
Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–40.
DeHaven KE, Sebastianelli WJ. Open meniscus repair. Indications, technique, and results. Clin Sports Med. 1990;9:577–87.
Harston A, Nyland J, Brand E, McGinnis M, Caborn DNM. Collagen meniscus implantation: a systematic review including rehabilitation and return to sports activity. Knee Surg Sports Traumatol Arthrosc. 2012;20:135–46.
Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23.
Chiari C, Koller U, Dorotka R, Eder C, Plasenzotti R, Lang S, et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr Cartil. 2006;14:1056–65.
Marijnissen WJCM, van Osch GJVM, Aigner J, van der Veen SW, Hollander AP, Verwoerd-Verhoef HL, et al. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23:1511–7.
Cook JL, Fox DB, Malaviya P, Tomlinson JL, Kuroki K, Cook CR, et al. Long-term outcome for large meniscal defects treated with small intestinal submucosa in a dog model. Am J Sports Med. 2006;34:32–42.
Heijkants RGJC, van Calck RV, de Groot JH, Pennings AJ, Schouten AJ, van Tienen TG, et al. Design, synthesis and properties of a degradable polyurethane scaffold for meniscus regeneration. J Mater Sci Mater Med. 2004;15:423–7.
Grande D, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–20.
Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Science. Transl Med. 2014;6:266ra171.
Webber RJ, Harris MG, Hough AJ. Cell culture of rabbit meniscal fibrochondrocytes: proliferative and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3:36–42.
Tumia NS, Johnstone AJ. Promoting the proliferative and synthetic activity of knee meniscal fibrochondrocytes using basic fibroblast growth factor in vitro. Am J Sports Med. 2004;32:915–20.
Kang S-W, Son S-M, Lee J-S, Lee E-S, Lee K-Y, Park S-G, et al. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J Biomed Mater Res A. 2006;78A:638–51.
Peretti GM, Gill TJ, Xu J-W, Randolph MA, Morse KR, Zaleske DJ. Cell-based therapy for meniscal repair. Am J Sports Med. 2004;32:146–58.
Esposito AR, Moda M, Cattani SM, de Santana GM, Barbieri JA, Munhoz MM, et al. PLDLA/PCL-T scaffold for meniscus tissue engineering. BioResearch open access. 2013;2:138–47.
Gunja NJ, Athanasiou KA. Additive and synergistic effects of bFGF and hypoxia on leporine meniscus cell-seeded PLLA scaffolds. J Tissue Eng Regen Med. 2010;4:115–22.
Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13:1103–12.
Fox DB, Warnock JJ, Stoker AM, Luther JK, Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res Vet Sci. 2010;88:326–32.
Ballard GA, Warnock JJ, Bobe G, Duesterdieck-Zellmer KF, Baker L, Baltzer WI, et al. Comparison of meniscal fibrochondrocyte and synoviocyte bioscaffolds toward meniscal tissue engineering in the dog. Res Vet Sci. 2014;97:400–8.
Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A. 2010;94A:1150–61.
Zellner J, Hierl K, Mueller M, Pfeifer C, Berner A, Dienstknecht T, et al. Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J Biomed Mater Res B Appl Biomater. 2013;101:1133–42.
Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, et al. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 2014;43:255–65.
Vangsness CTJ, Farr JI, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind controlled study. JBJS. 2014;96:90–8.
Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, et al. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878–87.
Horie M, Driscoll MD, Sampson HW, Sekiya I, Caroom CT, Prockop DJ, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. JBJS. 2012;94:701–12.
Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr Cartil. 2015;23:1007–17.
Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed Res Int. 2014;2014:10.
Mandal BB, Park S-H, Gil ES, Kaplan DL. Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials. 2011;32:639–51.
Halili AN, Hasirci N, Hasirci V. A multilayer tissue engineered meniscus substitute. J Mater Sci Mater Med. 2014;25:1195–209.
Tan G-K, Dinnes DLM, Butler LN, Cooper-White JJ. Interactions between meniscal cells and a self assembled biomimetic surface composed of hyaluronic acid, chitosan and meniscal extracellular matrix molecules. Biomaterials. 2010;31:6104–18.
Jakob M, Demarteau O, Schäfer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–77.
Baker BM, Nathan AS, Huffman GR, Mauck RL. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthr Cartil. 2009;17:336–45.
Port J, Jackson DW, Lee TQ, Simon TM. Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. Am J Sports Med. 1996;24:547–55.
Walsh CJ, Goodman D, Caplan AI, Goldberg VM. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999;5:327–37.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis & Rheumatism. 2003;48:3464–74.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84.
Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217–23.
Ding Z, Huang H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: superiority of meniscus as a cell source for meniscus repair. BMC Musculoskelet Disord. 2015;16:65.
Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–58.
Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends in Biotechnology. 2007;25:24–32.
Wyles SP, Yamada S, Oommen S, Maleszewski JJ, Beraldi R, Martinez-Fernandez A, et al. Inhibition of DNA topoisomerase II selectively reduces the threat of tumorigenicity following induced pluripotent stem cell-based myocardial therapy. Stem Cells Dev. 2014;23:2274–82.
Friedenstein AJ, Gorskaja J, Kulagina N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74.
Wegmeyer H, Bröske A-M, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606–18.
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52.
Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis & Rheumatism. 2002;46:704–13.
Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng A. 2012;18:1161–70.
Gawlitta D, van Rijen MH, Schrijver EJ, Alblas J, Dhert WJ. Hypoxia impedes hypertrophic chondrogenesis of human multipotent stromal cells. Tissue Eng Part A. 2012;18:1957–66.
Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochemical and Biophysical Research Communications. 417:305–10.
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Tasso R, Augello A, Carida’ M, Postiglione F, Tibiletti MG, Bernasconi B, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150–7.
Funding
The authors gratefully acknowledge funding from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences, NIH R01AR063698, and NIH DP1 AR068147.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narayanan, G., Bhattacharjee, M., Nair, L.S. et al. Musculoskeletal Tissue Regeneration: the Role of the Stem Cells. Regen. Eng. Transl. Med. 3, 133–165 (2017). https://doi.org/10.1007/s40883-017-0036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-017-0036-9