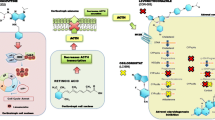
Abstract
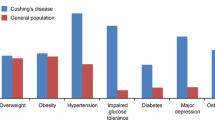
Endogenous Cushing’s syndrome is a chronic disease associated with increased morbidity and mortality if not appropriately treated. Recurrence and/or persistence of hypercortisolemia after surgical treatment, especially for Cushing’s disease, are high, and long-term medical treatment is used to decrease cortisol levels and risk of metabolic comorbidities. Medical treatment is also often required while waiting for radiation effects to take place. In some cases, severe or life-threatening hypercortisolism must be urgently and medically treated, via intravenous medications or with combination therapy, before patients can undergo surgery. In the last decade, medical treatment has progressed from a few steroidogenesis inhibitors to three novel drug groups: new inhibitors for steroidogenic enzymes with possibly fewer side effects, pituitary-directed drugs that aim to inhibit the pathophysiological pathways of Cushing’s disease, and glucocorticoid receptor antagonists that block cortisol’s action. Understanding the pathophysiology of Cushing’s syndrome has also led to the identification of potential targets that may decrease adrenocorticotrophic hormone and/or cortisol excess, and/or decrease tumor cell proliferation, and induce senescence or apoptosis. We provide here a review of current and near-future medical options to treat Cushing’s syndrome, and discuss updates on clinical trials and the efficacy and safety of novel or in-development drugs, as well as future potential targets.

Similar content being viewed by others
References
Loriaux DL. Diagnosis and differential diagnosis of Cushing’s syndrome. N Engl J Med. 2017;376:1451–9. https://doi.org/10.1056/NEJMra1505550.
Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and risk of venous thromboembolic events in endogenous Cushing’s syndrome: a systematic meta-analysis. Front Endocrinol. 2019. https://doi.org/10.3389/fendo.2018.00805.
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593–602. https://doi.org/10.1210/jc.2003-030871.
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366:914–24. https://doi.org/10.1056/NEJMoa1105743.
Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994. https://doi.org/10.1111/j.1365-2265.1994.tb02486.x.
Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocr Rev. 2015. http://dx.doi.org/10.1210/er.2013-1048.
Budyal S, Lila AR, Jalali R, Gupta T, Kasliwal R, Jagtap VS, et al. Encouraging efficacy of modern conformal fractionated radiotherapy in patients with uncured Cushing’s disease. Pituitary. 2014;17(1):60–7. https://doi.org/10.1007/s11102-013-0466-4.
Grant RA, Whicker M, Lleva R, Knisely JP, Inzucchi SE, Chiang VL. Efficacy and safety of higher dose stereotactic radiosurgery for functional pituitary adenomas: a preliminary report. World Neurosurg. 2014;82(1–2):195–201. https://doi.org/10.1016/j.wneu.2013.01.127.
Wilson PJ, Williams JR, Smee RI. Nelson’s syndrome: single centre experience using the linear accelerator (LINAC) for stereotactic radiosurgery and fractionated stereotactic radiotherapy. J Clin Neurosci. 2014;21(9):1520–4. https://doi.org/10.1016/j.jocn.2013.12.026.
Wilson PJ, Williams JR, Smee RI. Cushing’s disease: a single centre’s experience using the linear accelerator (LINAC) for stereotactic radiosurgery and fractionated stereotactic radiotherapy. J Clin Neurosci. 2014;21(1):100–6. https://doi.org/10.1016/j.jocn.2013.04.007.
Wattson DA, Tanguturi SK, Spiegel DY, Niemierko A, Biller BM, Nachtigall LB, et al. Outcomes of proton therapy for patients with functional pituitary adenomas. Int J Radiat Oncol Biol Phys. 2014;90(3):532–9. https://doi.org/10.1016/j.ijrobp.2014.06.068.
Hinojosa Amaya JM, Mireya Perez C, Villanueva Rodríguez LG, Avendano Vazquez E, Coronel Manzo DA, Vega Beyhart A et al. Effectivity and side effects of LINAC stereotactic radiotherapy as treatment for pituitary adenomas. 2018. ENDO 2018; March 17, 2018; SAT-626-LB; Chicago, IL2018.
Burman P, van Beek AP, Biller BM, Camacho-Hubner C, Mattsson AF. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J Clin Endocrinol Metab. 2017;102(3):1051–8. https://doi.org/10.1210/jc.2016-3402.
van Varsseveld NC, van Bunderen CC, Ubachs DH, Franken AA, Koppeschaar HP, van der Lely AJ, et al. Cerebrovascular events, secondary intracranial tumors, and mortality after radiotherapy for nonfunctioning pituitary adenomas: a subanalysis from the Dutch National Registry of Growth Hormone Treatment in Adults. J Clin Endocrinol Metab. 2015;100(3):1104–12. https://doi.org/10.1210/jc.2014-3697.
Yamanaka R, Abe E, Sato T, Hayano A, Takashima Y. Secondary intracranial tumors following radiotherapy for pituitary adenomas: a systematic review. Cancers (Basel). 2017;9:8. https://doi.org/10.3390/cancers9080103.
Valassi E, Aulinas A, Glad CA, Johannsson G, Ragnarsson O, Webb SM. A polymorphism in the CYP17A1 gene influences the therapeutic response to steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol (Oxf). 2017. https://doi.org/10.1111/cen.13414.
Cuevas-Ramos D, Fleseriu M. Treatment of Cushing’s disease: a mechanistic update. J Endocrinol. 2014;223:R19–39. https://doi.org/10.1530/JOE-14-0300.
Engelhardt D, Mann K, Hörmann R, Braun S, Karl HJ. Ketoconazole inhibits cortisol secretion of an adrenal adenoma in vivo and in vitro. Klin Wochenschr. 1983. https://doi.org/10.1007/bf01485030.
Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, et al. Ketoconazole in Cushing’s disease: is it worth a try. J Clin Endocrinol Metab. 2014;99:1623–30. https://doi.org/10.1210/jc.2013-3628.
Novotná A, Krasulová K, Bartoňková I, Korhoňová M, Bachleda P, Anzenbacher P, et al. Dual effects of ketoconazole cis-enantiomers on CYP3A4 in human hepatocytes and HepG2 cells. PLoS One. 2014;9:1–8. https://doi.org/10.1371/journal.pone.0111286.
Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J Mol Endocrinol. 2014;52. https://doi.org/10.1530/jme-14-0011.
Cuevas-Ramos D, Lim DST, Fleseriu M. Update on medical treatment for Cushing’s disease. Clinical Diabetes and Endocrinology. 2016;2:16. https://doi.org/10.1186/s40842-016-0033-9.
Karl Stalla G, Stalla J, Huber M, Loeffler JP, Höllt V, Von Werder K et al. Ketoconazole inhibits corticotropic cell function in vitro*. Endocrinology. 1988. https://doi.org/10.1210/endo-122-2-618.
Young J, Bertherat J, Vantyghem MC, Chabre O, Senoussi S, Chadarevian R, et al. Hepatic safety of ketoconazole in Cushing’s syndrome: results of a compassionate use programme in France. Eur J Endocrinol. 2018;178:447–58. https://doi.org/10.1530/EJE-17-0886.
Ollivier M, Haissaguerre M, Ferriere A, Tabarin A. Should we avoid using ketoconazole in patients with severe Cushing’s syndrome and increased levels of liver enzymes? Eur J Endocrinol. 2018;179:L1–2. https://doi.org/10.1530/EJE-18-0694.
Sobh M, el-Agroudy A, Moustafa F, Harras F, el-Bedewy M, Ghoneim M. Coadministration of ketoconazole to cyclosporin-treated kidney transplant recipients: a prospective randomized study. Am J Nephrol. 1995;15(6):493–9. https://doi.org/10.1159/000168892.
Charles BG, Ravenscroft PJ, Rigby RJ. The ketoconazole-cyclosporin interaction in an elderly renal transplant patient. Aust N Z J Med. 1989;19(3):292–3.
Hwang WL, Gau JP, Young JH, Chia LG. Ketoconazole and high-dose methylprednisolone predisposing to cyclosporine-induced seizures: report of 3 cases. Acta Haematol. 1992;88(2–3):139–41. https://doi.org/10.1159/000204670.
Itakura H, Vaughn D, Haller DG, O’Dwyer PJ. Rhabdomyolysis from cytochrome p-450 interaction of ketoconazole and simvastatin in prostate cancer. J Urol. 2003;169(2):613. https://doi.org/10.1097/01.ju.0000043761.61046.b0.
Watkins JL, Atkinson BJ, Pagliaro LC. Rhabdomyolysis in a prostate cancer patient taking ketoconazole and simvastatin: case report and review of the literature. Ann Pharmacother. 2011;45(2):e9. https://doi.org/10.1345/aph.1P433.
Backman JT, Kivistö KT, Olkkola KT, Neuvonen PJ. The area under the plasma concentration–time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol. 1998;54(1):53–8. https://doi.org/10.1007/s002280050420.
Greenblatt DJ, von Moltke LL, Harmatz JS, Harrel LM, Tobias S, Shader RI, et al. Interaction of triazolam and ketoconazole. Lancet. 1995;345(8943):191.
Wrighton SA, Ring BJ. Inhibition of human CYP3A catalyzed 1’-hydroxy midazolam formation by ketoconazole, nifedipine, erythromycin, cimetidine, and nizatidine. Pharm Res. 1994;11(6):921–4.
Riedl M, Maier C, Zettinig G, Nowotny P, Schima W, Luger A. Long term control of hypercortisolism with fluconazole: case report and in vitro studies. Eur J Endocrinol. 2006. https://doi.org/10.1530/eje.1.02120.
Van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J et al. Fluconazole inhibits human adrenocortical steroidogenesis in vitro. J Endocrinol. 2012. https://doi.org/10.1530/joe-12-0310.
Heyn J, Geiger C, Hinske CL, Briegel J, Weis F. Medical suppression of hypercortisolemia in Cushing's syndrome with particular consideration of etomidate. Pituitary. 2012;15:117–25. https://doi.org/10.1007/s11102-011-0314-3.
Preda VA, Sen J, Karavitaki N, Grossman AB. Etomidate in the management of hypercortisolaemia in Cushing's syndrome: a review. Eur J Endocrinol. 2012;167:137–43. https://doi.org/10.1530/EJE-12-0274.
Allolio B, Schulte HM, Kaulen D, Reincke M, Jaursch-Hancke C, Winkelmann W. Nonhypnotic low-dose etomidate for rapid correction of hypercortisolaemia in Cushing’s syndrome. Klin Wochenschr. 1988. https://doi.org/10.1007/bf01735795.
Schulte HM, Benker G, Reinwein D, Sippell WG, Allolio B. Infusion of low dose etomidate: correction of hypercortisolemia in patients with Cushing’s syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab. 1990. https://doi.org/10.1210/jcem-70-5-1426.
Carroll TB, Peppard WJ, Herrmann DJ, Javorsky BR, Wang TS, Patel H, et al. Continuous etomidate infusion for the management of severe Cushing syndrome: validation of a standard protocol. J Endocrine Soc. 2019;3:1–12. https://doi.org/10.1210/js.2018-00269.
Liddle GW, Estep HL, Kendall JWJ, Williams WCJ, Townes AW. Clinical application of a new test of pituitary reserve*. J Clin Endocrinol Metabol. 1959;19(8):875–94. https://doi.org/10.1210/jcem-19-8-875.
Gower DB. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem. 1974. https://doi.org/10.1016/0022-4731(74)90051-x.
Fleseriu M. Medical management of persistent and recurrent Cushing disease. Neurosurg Clin N Am. 2012;23:653–68. https://doi.org/10.1016/j.nec.2012.06.012.
Nieman LK, Chrousos GP, Kellner C, Spitz IM, Nisula BC, Cutler GB et al. Successful treatment of Cushing’s syndrome with the glucocorticoid antagonist RU 486. J Clin Endocrinol Metabol. 1985. https://doi.org/10.1210/jcem-61-3-536.
Verhelst JA, Trainer PJ, Howlett TA, Perry L, Rees LH, Grossman AB et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing’s syndrome. Clin Endocrinol (Oxf). 1991. https://doi.org/10.1111/j.1365-2265.1991.tb03517.x.
Kamenický P, Droumaguet C, Salenave S, Blanchard A, Jublanc C, Gautier JF et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2011. https://doi.org/10.1210/jc.2011-0536.
Kamenicky P, Droumaguet C, Baudin E, Salenave S, Trabado S, Cazabat L, et al. Combined anticortisolic therapy by metyrapone, ketoconazole and mitotane: an alternative to adrenalectomy in severe Cushing's syndrome, in Endocrine Abstracts. Prague, Czech Republic 12th European Congress of Endocrinology, 2010 22 OC4.4.
Ceccato F, Zilio M, Barbot M, Albiger N, Antonelli G, Plebani M, et al. Metyrapone treatment in Cushing’s syndrome: a real-life study. Endocrine. 2018;62(3):701–11. https://doi.org/10.1007/s12020-018-1675-4.
Drugs.com. Metyrapone drug interactions. 2019. https://www.drugs.com/drug-interactions/metyrapone.html. Accessed 28 Mar 2019
Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metabol. 2005. https://doi.org/10.1210/jc.2004-2361.
Boscaro M, Barzon L, Fallo F, Sonino N. Cushing’s syndrome. Lancet. 2001. https://doi.org/10.1016/s0140-6736(00)04172-6.
Cueto C, Brown JH, Richardson AP Jr. Biological studies on an adrenocorticolytic agent and the isolation of the active components. Endocrinology. 1958;62(3):334–9. https://doi.org/10.1210/endo-62-3-334.
Van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Den Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011. https://doi.org/10.1530/eje-10-0956.
Baudry C, Coste J, Khalil RB, Silvera S, Guignat L, Guibourdenche J et al. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol. 2012. https://doi.org/10.1530/eje-12-0358.
Motte-Signoret E, Rothenbuhler A, Gaillard S, Lahlou N, Teinturier C, Coutant R et al. Mitotane (op’DDD) restores growth and puberty in nine children with Cushing’s disease. Endocrine Connect. 2018. https://doi.org/10.1530/ec-18-0215.
Robinson BG, Hales IB, Henniker AJ, Ho K, Luttrell BM, Smee IR, et al. The effect of o, p’-DDD on adrenal steroid replacement therapy requirements. Clin Endocrinol (Oxf). 1987;27(4):437–44.
Drugs.com. Mitotane Drug Interactions. 2019. https://www.drugs.com/drug-interactions/mitotane-index.html. Accessed 28 Mar 2019
Bertagna X, Pivonello R, Fleseriu M, Zhang Y, Robinson P, Taylor A, et al. LCI699, a Potent 11β-hydroxylase Inhibitor, normalizes urinary cortisol in patients with Cushing’s disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab. 2014;99:1375–83. https://doi.org/10.1210/jc.2013-2117.
Amar L, Azizi M, Menard J, Peyrard S, Watson C, Plouin PF. Aldosterone synthase inhibition with LCI699: A proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010. https://doi.org/10.1161/hypertensionaha.110.157271.
Calhoun DA, White WB, Krum H, Guo W, Bermann G, Trapani A et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: Results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation. 2011. https://doi.org/10.1161/circulationaha.111.029892.
Fleseriu M, Pivonello R, Young J, Hamrahian AH, Molitch ME, Shimizu C, et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary. 2016;19:138–48. https://doi.org/10.1007/s11102-015-0692-z.
Biller BM, Newell-Price J, Fleseriu M, Bertagna X, Findling J, Shimatsu A et al. OR16-2. Osilodrostat treatment in Cushing’s disease (CD): results from a phase III, multicenter, double-blind, randomized withdrawal study (LINC 3). ENDO 2019; March 24, 2019; Session OR16–OR16. Pituitary and neuroendocrine clinical trials and studies; New Orleans, LA2019.
Fleseriu M, Castinetti F. Updates on the role of adrenal steroidogenesis inhibitors in Cushing’s syndrome: a focus on novel therapies. Pituitary. 2016;19:643–53. https://doi.org/10.1007/s11102-016-0742-1.
Feelders RA, Kadioglu P, Bex MA, Devia DG, Boguszewski CL, Yavus DG et al., editors. Prospective phase II study (CAPACITY) of pasireotide monotherapy or in combination with cabergoline in patients with Cushing’s disease. In: Endocrine Society Meeting 2017; 2017 04/03/2017; Orlando, FL.
Salvatori R, DelConte A, Geer EB, Koziol T, Jorkasky D. An open-label study to assess the safety and efficacy of levoketoconazole (COR-003) in the treatment of endogenous Cushing’s syndrome. In: Endocrine Society Meeting; 05/06/2015; San Diego, CA2015.
th CONGRESS of the European NeuroEndocrine Association. Scientific Program. 2018. http://www.enea2018.com/program.html. Accessed 03/28/2019 2019.
Langlois DK, Fritz MC, Schall WD, Bari Olivier N, Smedley RC, Pearson PG, et al. ATR-101, a selective ACAT1 inhibitor, decreases ACTH-stimulated cortisol concentrations in dogs with naturally occurring Cushing’s syndrome. BMC Endocr Disord. 2018;18:1–12. https://doi.org/10.1186/s12902-018-0251-5.
Sanders K, de Wit WL, Mol JA, Kurlbaum M, Kendl S, Kroiss M, et al. Abiraterone acetate for Cushing syndrome: study in a canine primary adrenocortical cell culture model. Endocrinology. 2018;159:3689–98. https://doi.org/10.1210/en.2018-00588.
Markey KA, Ottridge R, Mitchell JL, Rick C, Woolley R, Ives N, et al. Assessing the efficacy and safety of an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor (AZD4017) in the Idiopathic Intracranial Hypertension Drug Trial, IIH:DT: Clinical Methods and Design for a Phase II Randomized Controlled Trial. JMIR Res Protoc. 2017;6(9):e181. https://doi.org/10.2196/resprot.7806.
de Bruin C, Feelders RA, Waaijers AM, van Koetsveld PM, Sprij-Mooij DM, Lamberts SWJ, et al. Differential regulation of human dopamine D2 and somatostatin receptor subtype expression by glucocorticoids in vitro. J Mol Endocrinol. 2009;42:47–56. https://doi.org/10.1677/JME-08-0110.
Tateno T, Kato M, Tani Y, Oyama K, Yamada S, Hirata Y. Differential expression of somatostatin and dopamine receptor subtype genes in adrenocorticotropin (ACTH)-secreting pituitary tumors and silent corticotroph adenomas. Endocr J. 2009;56(4):579–84.
Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98:3446–53. https://doi.org/10.1210/jc.2013-1771.
Colao A, De Block C, Gaztambide MS, Kumar S, Seufert J, Casanueva FF. Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary. 2014. https://doi.org/10.1007/s11102-013-0483-3.
Pivonello R, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a phase III study. Clin Endocrinol (Oxf). 2014;81:408–17. https://doi.org/10.1111/cen.12431.
Schopohl J, Gu F, Rubens R, Van Gaal L, Bertherat J, Ligueros-Saylan M, et al. Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: results from an open-ended, open-label extension trial. Pituitary. 2015;18:604–12. https://doi.org/10.1007/s11102-014-0618-1.
Fleseriu M, Petersenn S, Biller BMK, Kadioglu P, De Block C, T’Sjoen G et al. Long-term efficacy and safety of once-monthly pasireotide in patients with Cushing’s disease: a phase III extension study. In: 20th European Congress of Endocrinology 19–22 May 2018 Barcelona, Spain: European Society of Endocrinology 2018.
Lacroix A, Gu F, Gallardo W, Pivonello R, Yu Y, Witek P, et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 2018;6(1):17–26. https://doi.org/10.1016/s2213-8587(17)30326-1.
Witek P, Biller BMK, Lacroix A, Feelders R, Li Y, Geer EB et al. Predictors of response to long-acting pasireotide in patients with Cushing’s disease during a phase III study. In: 20th European Congress of Endocrinology 19–22 May 2018 Barcelona, Spain: European Society of Endocrinology 2018.
Newell-Price J, Pivonello R, Tabarin A, Fleseriu M, Witek P, Gadelha M et al. Late-night salivary cortisol levels in a phase III study of long-acting pasireotide in patients with Cushing’s disease. In: 20th European Congress of Endocrinology 19–22 May 2018 Barcelona, Spain: European Society of Endocrinology 2018.
Pivonello R, Ferone D, De Herder WW, Kros JM, Del Basso De Caro ML, Arvigo M et al. Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab. 2004;89:2452-62. https://doi.org/10.1210/jc.2003-030837.
Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A. Cabergoline monotherapy in the long-term treatment of Cushing’s disease. European Journal of Endocrinology. 2010. https://doi.org/10.1530/eje-10-0382.
Burman P, Edén-Engström B, Ekman B, Karlsson FA, Schwarcz E, Wahlberg J. Limited value of cabergoline in Cushing’s disease: a prospective study of a 6-week treatment in 20 patients. Eur J Endocrinol. 2016. https://doi.org/10.1530/eje-15-0807.
Ferriere A, Cortet C, Chanson P, Delemer B, Caron P, Chabre O et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol. 2017. https://doi.org/10.1530/eje-16-0662.
Barbot M, Albiger N, Ceccato F, Zilio M, Frigo AC, Denaro L et al. Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment. Should we start with cabergoline or ketoconazole? Pituitary. 2014;17:109-17. https://doi.org/10.1007/s11102-013-0475-3.
Auriemma RS, Pivonello R, Ferreri L, Priscitelli P, Colao A. Cabergoline use for pituitary tumors and valvular disorders. Endocrinol Metab Clin North Am. 2015;44(1):89–97. https://doi.org/10.1016/j.ecl.2014.10.007.
Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with Cabergoline. J Clin Endocrinol Metab. 2018. https://doi.org/10.1210/jc.2018-01071.
Halevy C, Whitelaw BC. How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary. 2017;20:261–6. https://doi.org/10.1007/s11102-016-0745-y.
Matsuno A, Murakami M, Hoya K, Yamada SM, Miyamoto S, Yamada S, et al. Molecular status of pituitary carcinoma and atypical adenoma that contributes the effectiveness of temozolomide. Med Mol Morphol. 2014;47:1–7. https://doi.org/10.1007/s00795-013-0050-z.
Dillard TH, Gultekin SH, Delashaw JB, Yedinak CG, Neuwelt EA, Fleseriu M. Temozolomide for corticotroph pituitary adenomas refractory to standard therapy. Pituitary. 2011;14:80–91. https://doi.org/10.1007/s11102-010-0264-1.
Annamalai AK, Dean AF, Kandasamy N, Kovacs K, Burton H, Halsall DJ, et al. Temozolomide responsiveness in aggressive corticotroph tumours: a case report and review of the literature. Pituitary. 2012;15:276–87. https://doi.org/10.1007/s11102-011-0363-7.
Bengtsson D, Schrøder HD, Andersen M, Maiter D, Berinder K, Rasmussen UF, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100:1689–98. https://doi.org/10.1210/jc.2014-4350.
Losa M, Bogazzi F, Cannavo S, Ceccato F, Curtò L, De Marinis L, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126:519–25. https://doi.org/10.1007/s11060-015-1991-y.
Lasolle H, Cortet C, Castinetti F, Cloix L, Caron P, Delemer B, et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol. 2017;176:769–77. https://doi.org/10.1530/EJE-16-0979.
Zacharia BE, Gulati AP, Bruce JN, Carminucci AS, Wardlaw SL, Siegelin M, et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery. 2014;74:447–55. https://doi.org/10.1227/NEU.0000000000000251.
Langlois F, Chu J, Fleseriu M. Pituitary-directed therapies for Cushing’s disease. Front Endocrinol (Lausanne). 2018. https://doi.org/10.3389/fendo.2018.00164.
Fuertes M, Tkatch J, Rosmino J, Nieto L, Guitelman MA, Arzt E. New insights in Cushing disease treatment with focus on a derivative of vitamin A. Front Endocrinol (Lausanne). 2018;9:1–12. https://doi.org/10.3389/fendo.2018.00262.
Páez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E et al. Retinoic acid prevents experimental Cushing syndrome. J Clin Investig. 2001. https://doi.org/10.1172/jci11098.
Castillo V, Giacomini D, Páez-Pereda M, Stalla J, Labeur M, Theodoropoulou M et al. Retinoic acid as a novel medical therapy for Cushing’s disease in dogs. Endocrinology. 2006. https://doi.org/10.1210/en.2006-0414.
Vilar L, Albuquerque JL, Lyra R, Trovão Diniz E, Rangel Filho F, Gadelha P, et al. The role of isotretinoin therapy for Cushing’s disease: results of a prospective study. Int J Endocrinol. 2016;2016:15–8. https://doi.org/10.1155/2016/8173182.
Pecori Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Corsello SM et al. Potential Role for Retinoic Acid in Patients with Cushing’s disease. J Clin Endocrinol Metabol. 2012;97:3577-83. https://doi.org/10.1210/jc.2012-2328.
Liu NA, Araki T, Cuevas-Ramos D, Hong J, Ben-Shlomo A, Tone Y, et al. Cyclin e-mediated human proopiomelanocortin regulation as a therapeutic target for Cushing disease. J Clin Endocrinol Metab. 2015;100:2557–64. https://doi.org/10.1210/jc.2015-1606.
Liu N-A, Jiang H, Ben-Shlomo A, Wawrowsky K, Fan X-M, Lin S, et al. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc Natl Acad Sci. 2011;108:8414–9. https://doi.org/10.1073/pnas.1018091108.
Araki T, Liu NA, Tone Y, Cuevas-Ramos D, Heltsley R, Tone M, et al. E2F1-mediated human POMC expression in ectopic Cushing’s syndrome. Endocr Relat Cancer. 2016;23:857–70. https://doi.org/10.1530/ERC-16-0206.
Benson C, White J, De Bono J, O’Donnell A, Raynaud F, Cruickshank C et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007. https://doi.org/10.1038/sj.bjc.6603509.
Le Tourneau C, Faivre S, Laurence V, Delbaldo C, Vera K, Girre V et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer. 2010. https://doi.org/10.1016/j.ejca.2010.08.001.
Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121:4712–21. https://doi.org/10.1172/JCI60417.
Araki T, Liu X, Kameda H, Tone Y. EGFR Induces E2F1-mediated corticotroph tumorigenesis. J Endocrine Soc. 2017;1:127–43. https://doi.org/10.1210/js.2016-1053.
Onguru O, Scheithauer BW, Kovacs K, Vidal S, Jin L, Zhang S et al. Analysis of epidermal growth factor receptor and activated epidermal growth factor receptor expression in pituitary adenomas and carcinomas. Modern Pathol. https://doi.org/10.1038/modpathol.3800118.
Ben-Shlomo A, Cooper O. Role of tyrosine kinase inhibitors in the treatment of pituitary tumours: from bench to bedside. Curr Opin Endocrinol Diabetes Obesity. 2017;24:301–5. https://doi.org/10.1097/MED.0000000000000344.
Langlois F, Mccartney S, Fleseriu M. Recent progress in the medical therapy of pituitary tumors. Endocrinol Metab. 2017;3232162:162–70. https://doi.org/10.3803/EnM.2017.32.2.162.
Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25:306–17. https://doi.org/10.1038/cr.2015.20.
Hayashi K, Inoshita N, Kawaguchi K, Ardisasmita AI, Suzuki H, Fukuhara N, et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol. 2016;174:213–26. https://doi.org/10.1530/EJE-15-0689.
Jian FF, Li YF, Chen YF, Jiang H, Chen X, Zheng LL, et al. Inhibition of ubiquitin-specific peptidase 8 suppresses adrenocorticotropic hormone production and tumorous corticotroph cell growth in AtT20 cells. Chin Med J. 2016;129:2102–8. https://doi.org/10.4103/0366-6999.189047.
Chen J, Jian X, Deng S, Ma Z, Shou X, Shen Y, et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nature Communications. 2018;9:1–9. https://doi.org/10.1038/s41467-018-05275-5.
Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay J-Y, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–36. https://doi.org/10.1056/NEJMoa1502309.
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600–mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. https://doi.org/10.1056/NEJMoa1112302.
Riebold M, Kozany C, Freiburger L, Sattler M, Buchfelder M, Hausch F, et al. A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat Med. 2015;21:276–80. https://doi.org/10.1038/nm.3776.
Mengs U, Torsten Pohl R-, Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012;13:1964–70. https://doi.org/10.2174/138920112802273353.
Tiwari P, Mishra K. Silibinin in cancer therapy: a promising prospect. Cancer Res Front. 2015;1:303–18. https://doi.org/10.17980/2015.303.
Du L, Bergsneider M, Mirsadraei L, Young SH, Jonker JW, Downes M, et al. Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc Natl Acad Sci. 2013;110:8555–60. https://doi.org/10.1073/pnas.1306182110.
Zhang D, Du L, Heaney AP. Testicular receptor-4: novel regulator of glucocorticoid resistance. J Clin Endocrinol Metab. 2016. https://doi.org/10.1210/jc.2016-1379.
Feldhaus AL, Anderson K, Dutzar B, Ojala E, McNeill PD, Fan P, et al. ALD1613, a novel long-actingmonoclonal antibody to control ACTH-driven pharmacology. Endocrinology. 2017;158:1–8. https://doi.org/10.1210/en.2016-1455.
Robertson S, Diver LA, Alvarez-Madrazo S, Livie C, Ejaz A, Fraser R et al. Regulation of corticosteroidogenic genes by microRNAs. Int J Endocrinol. 2017. https://doi.org/10.1155/2017/2021903.
Di Ieva A, Butz H, Niamah M, Rotondo F, De Rosa S, Sav A et al. MicroRNAs as biomarkers in pituitary tumors. Neurosurgery. 2014. https://doi.org/10.1227/neu.0000000000000369.
Amaral FC, Torres N, Saggioro F, Neder L, Machado HR, Silva WA et al. MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J Clin Endocrinol Metab. 2009. https://doi.org/10.1210/jc.2008-1451.
Belaya Z, Khandaeva P, Nikitin A, Solodovnikov A, Sitkin I, Grebennikova T et al. Plasma microRNA expression in patients with Cushing’s disease differs from ACTH-ectopic Cushing’s syndrome. In: 20th European Congress of Endocrinology 19–22 May 2018 Barcelona, Spain: European Society of Endocrinology 2018.
Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Res. 1996;17:245–61.
Newfield RS, Spitz IM, Isacson C, New MI. Long-term mifepristone (RU486) therapy resulting in massive benign endometrial hyperplasia. Clin Endocrinol (Oxf). 2001. https://doi.org/10.1046/j.1365-2265.2001.01026.x.
Steinauer J, Pritts EA, Jackson R, Jacoby AF. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004;103:1331–6. https://doi.org/10.1097/01.AOG.0000127622.63269.8b.
Bali U, Phillips T, Hunt H, Unitt J. FKBP5 mRNA expression is a biomarker for GR antagonism. J Clin Endocrinol Metab. 2016;101:4305–12. https://doi.org/10.1210/jc.2016-1624.
Carmichael JD, Fleseriu M. Mifepristone: Is there a place in the treatment of Cushing’s disease? Endocrine. 2013;44:20–32. https://doi.org/10.1007/s12020-012-9846-1.
Drugs.com. Mifepristone Drug Interactions. 2019. https://www.drugs.com/drug-interactions/mifepristone-index.html. Accessed 28 Mar 2019.
Hunt HJ, Belanoff JK, Walters I, Gourdet B, Thomas J, Barton N, et al. Identification of the clinical candidate (R)-(1-(4-fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone (CORT125134): a selective glucocorticoid receptor. J Med Chem. 2017;60:3405–21. https://doi.org/10.1021/acs.jmedchem.7b00162.
Hunt H, Donaldson K, Strem M, Zann V, Leung P, Sweet S et al. Assessment of safety, tolerability, pharmacokinetics, and pharmacological effect of orally administered CORT125134: an adaptive, double-blind, randomized, placebo-controlled phase 1 clinical study. Clinical Pharmacology in Drug Development. 2017;94025. https://doi.org/10.1002/cpdd.389.
Moraitis A, Agrawal N, Bancos I, Celi F, Gordon M, Kargi A, et al: Open-label phase 2 study to assess safety and efficacy of relacorilant (cort125134), a selective cortisol modulator, in the treatment of endogenous hypercortisolism in American Association of Clinical Endocrinologists, 2018. Boston, MA, 2018, p Abstract # 1219.
Valassi E, Crespo I, Gich I, Rodríguez J, Webb SM. A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol (Oxf). 2012. https://doi.org/10.1111/j.1365-2265.2012.04424.x.
Pivonello R, Kadioglu P, Bex M, Devia DG, Boguszewski C, Yavuz DG et al. Pasireotide alone or in combination with cabergoline effectively controls urinary free cortisol levels: results from a prospective study in patients with Cushing’s disease (CAPACITY). In: 19th European Congress of Endocrinology 20–23 May 2017 Lisbon, Portugal: European Society of Endocrinology 2017.
Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med. 2010;362(19):1846–8. https://doi.org/10.1056/NEJMc1000094.
Scaroni C, Regazzo D, Ceccato F, Albiger NM, Ferasin S, Occhi G, et al. Activation of the dopamine receptor type-2 (DRD2) promoter by 9-cis retinoic acid in a cellular model of Cushing’s disease mediates the inhibition of cell proliferation and ACTH secretion without a complete corticotroph-to-melanotroph transdifferentiation. Endocrinology. 2014;155(9):3538–49. https://doi.org/10.1210/en.2013-1820.
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454–62. https://doi.org/10.1210/jc.2007-2734.
Fleseriu M, Petersenn S. New avenues in the medical treatment of Cushing’s disease: corticotroph tumor targeted therapy. J Neurooncol. 2013;114:1–11. https://doi.org/10.1007/s11060-013-1151-1.
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(8):2807–31. https://doi.org/10.1210/jc.2015-1818.
Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Sen K, et al. High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2014;80(2):261–9. https://doi.org/10.1111/cen.12259.
Alexandraki KI, Grossman AB. Therapeutic strategies for the treatment of severe Cushing’s syndrome. Drugs. 2016;76(4):447–58. https://doi.org/10.1007/s40265-016-0539-6.
Acknowledgements
The authors thank Shirley McCartney, PhD, for manuscript preparation and editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
José Miguel Hinojosa-Amaya and Daniel Cuevas-Ramos have no conflicts of interest that are directly relevant to the content of this study. Maria Fleseriu is a principal investigator with funding from research grants to Oregon Health & Science University from Millendo, Novartis, and Strongbridge, and has done occasional scientific consulting work for Novartis and Strongbridge.
Rights and permissions
About this article
Cite this article
Hinojosa-Amaya, J.M., Cuevas-Ramos, D. & Fleseriu, M. Medical Management of Cushing’s Syndrome: Current and Emerging Treatments. Drugs 79, 935–956 (2019). https://doi.org/10.1007/s40265-019-01128-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01128-7