Abstract
Introduction
Eltrombopag and romiplostim are thrombopoietin receptor agonists (TPO-RAs) marketed for immune thrombocytopenia (ITP). Thrombotic events have been reported with both drugs. This study was aimed at assessing whether there is a signal for differential risks of thrombosis between these two TPO-RAs.
Methods
We carried out a disproportionality analysis in the World Health Organization global individual case safety report (ICSR) database (VigiBase®). We selected all ICSRs with exposure to a TPO-RA between January 2011 and December 2014. We searched for exposures to eltrombopag or romiplostim in thrombosis reports as compared with other ICSRs, and we calculated adjusted reporting odds ratios (aRORs).
Results
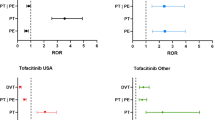
We identified 5850 ICSRs, including 764 cases of thrombosis. In multivariate analyses, there was a signal for an increased risk of thrombosis (venous or arterial; aROR 1.72, 95 % confidence interval [CI] 1.47–2.02), venous thrombosis (aROR 1.88, 95 % CI 1.53–2.31), arterial thrombosis (aROR 1.54, 95 % CI 1.18–2.00), ischaemic stroke (aROR 1.65, 95 % CI 1.13–2.42) and myocardial infarction (aROR 1.50, 95 % CI 1.05–2.13) with eltrombopag as compared with romiplostim. Restriction to ICSRs reported by physicians led to similar results. However, worldwide dispensing data for romiplostim and eltrombopag were not accessible, so the rates of thrombosis with both drugs were not normalized by the daily defined doses and the generalizability of the results is limited.
Conclusion
This study suggests the presence of a signal for an increased risk of thrombosis with eltrombopag compared with romiplostim. These results must be confirmed and quantified by large aetiological pharmacoepidemiological studies.

Similar content being viewed by others
References
Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365:734–41.
US Food and Drug Administration Center for Drug Evaluation and Research. Summary review, Nplate®. 2008. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. Accessed 30 Sept 2014.
US Food and Drug Administration Center for Drug Evaluation and Research. Summary review, Promacta®. 2008. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. Accessed 30 Sept 2014.
European Medicines Agency. Committee for Medicinal Products for Human Use assessment report for Nplate. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000942/WC500039475.pdf. Accessed 30 Sept 2014.
European Medicines Agency. Committee for Medicinal Products for Human Use assessment report for Revolade. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001110/WC500089967.pdf. Accessed 30 Sept 2014.
US Food and Drug Administration Departement of Health and Human Services. Supplement approval for Promacta. 2012. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo. Accessed 30 Sept 2014.
European Medicines Agency. CHMP group of an extension of marketing authorisation and variations assessment report, Revolade®. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001110/WC500152310.pdf. Accessed 30 Sept 2014.
Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403.
Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402.
Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121:537–45.
Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161:411–23.
Gernsheimer TB, George JN, Aledort LM, Tarantino MD, Sunkara U, Matthew Guo D, et al. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J Thromb Haemost. 2010;8:1372–82.
Khellaf M, Michel M, Quittet P, Viallard J-F, Alexis M, Roudot-Thoraval F, et al. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood. 2011;118:4338–45.
Garabet L, Lee S, Mowinckel M-C, Jonassen C, Liebman H, Bussel J, et al. Effect of thrombopoietin receptor agonists on coagulation and platelet activation in patients with immune thrombocytopenia. Haematologica. 2015;100:586.
Erhardt JA, Erickson-Miller CL, Aivado M, Abboud M, Pillarisetti K, Toomey JR. Comparative analyses of the small molecule thrombopoietin receptor agonist eltrombopag and thrombopoietin on in vitro platelet function. Exp Hematol. 2009;37:1030–7.
Khellaf M, Viallard J-F, Hamidou M, Cheze S, Roudot-Thoraval F, Lefrere F, et al. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica. 2013;98:881–7.
González-Porras JR, Mingot-Castellano ME, Andrade MM, Alonso R, Caparrós I, Arratibel MC, et al. Use of eltrombopag after romiplostim in primary immune thrombocytopenia. Br J Haematol. 2015;169(1):111–6.
Moulis G, Bagheri H, Sailler L, Jonville-Bera A-P, Weber E, Guy C, et al. Are adverse drug reaction patterns different between romiplostim and eltrombopag? 2009–2013 French pharmacovigilance assessment. Eur J Intern Med. 2014;25:777–80.
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–19.
World Health Organization Uppsala Monitoring Centre. Pharmacovigilance: ensuring the safe use of medicines—WHO policy perspectives on medicines. 2004. http://apps.who.int/medicinedocs/fr/d/Js6164e. Accessed 30 Sept 2014.
Brown EG, Wood L, Wood S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999;20:109–17.
International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Introductory guide for standardised MedDRA queries (SMQs). http://www.meddra.org/how-to-use/support-documentation/english. Accessed 30 Sept 2014.
Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72:905–8.
Thachil J, Callaghan T, Martlew V. Thromboembolic events are not uncommon in patients with immune thrombocytopenia. Br J Haematol. 2010;150:496–7.
Yusuf HR, Hooper WC, Beckman MG, Zhang QC, Tsai J, Ortel TL. Risk of venous thromboembolism among hospitalizations of adults with selected autoimmune diseases. J Thromb Thrombolysis. 2014;38:306–13.
Donnellan E, Kevane B, Bird BRH, Ainle FN. Cancer and venous thromboembolic disease: from molecular mechanisms to clinical management. Curr Oncol. 2014;21:134–43.
Paran D, Herishanu Y, Elkayam O, Shopin L, Ben-Ami R. Venous and arterial thrombosis following administration of intravenous immunoglobulins. Blood Coagul Fibrinolysis. 2005;16:313–8.
Stephen M. Introduction. Stephens’ detection and evaluation of adverse drug reactions. 6th ed. Chichester: Wiley; 2014. p. 14.
Durrieu G, Palmaro A, Pourcel L, Caillet C, Faucher A, Jacquet A, et al. First French experience of ADR reporting by patients after a mass immunization campaign with influenza A (H1N1) pandemic vaccines: a comparison of reports submitted by patients and healthcare professionals. Drug Saf. 2012;35:845–54.
Pages A, Bondon-Guitton E, Montastruc JL, Bagheri H. Undesirable effects related to oral antineoplastic drugs: comparison between patients’ internet narratives and a national pharmacovigilance database. Drug Saf. 2014;37:629–37.
Pierfitte C, Bégaud B, Lagnaoui R, Moore ND. Is reporting rate a good predictor of risks associated with drugs? Br J Clin Pharmacol. 1999;47:329–31.
Weber J. Epidemiology of adverse reactions to nonsteroidal antiinflammatory drugs. In: Rainsford KD, Velo GP, editors. Advances in inflammation research. New York: Raven; 1984. p. 1–7.
Haramburu F, Bégaud B, Moride Y. Temporal trends in spontaneous reporting of unlabelled adverse drug reactions. Br J Clin Pharmacol. 1997;44:299–301.
Moulis G, Sommet A, Durrieu G, Bagheri H, Lapeyre-Mestre M, Montastruc J-L, et al. Trends of reporting of “serious” vs. “non-serious” adverse drug reactions over time: a study in the French pharmacovigilance database. Br J Clin Pharmacol. 2012;74:201–4.
Acknowledgments
The authors thank the Uppsala Monitoring Centre, which provided and gave permission to use the data analysed in the present study. The opinions and conclusions in this study are not necessarily those of the various National Centres or of the World Health Organization. We also thank all of the pharmacovigilance centres worldwide, as well as Prof. Jean-Louis Montastruc for access to VigiBase®.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was academic (INSERM and the University of Toulouse). T.T.L. Nguyen received a grant from the Fondation Pierre Fabre for her stay in the UMR 1027 INSERM-University of Toulouse, where this work was carried out. The Fondation Pierre Fabre had no role in the subject, design, analysis, interpretation or manuscript drafting for this work.
Conflict of interest
Thi-Thanh Loan Nguyen, Aurore Palmaro, François Montastruc, Maryse Lapeyre-Mestre and Guillaume Moulis have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, TT.L., Palmaro, A., Montastruc, F. et al. Signal for Thrombosis with Eltrombopag and Romiplostim: A Disproportionality Analysis of Spontaneous Reports Within VigiBase® . Drug Saf 38, 1179–1186 (2015). https://doi.org/10.1007/s40264-015-0337-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0337-1