Abstract
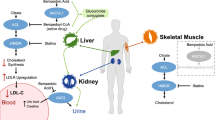
Statins are currently the first-line drugs for managing dyslipidemia due to their substantial clinical efficacy in reducing low-density lipoprotein cholesterol (LDL-C) and the risk of atherosclerotic cardiovascular disease (ASCVD). However, many patients do not reach their LDL-C target despite taking high-dose statins and some patients are intolerant of these drugs. Therefore, an additional or alternative pharmacological intervention may be required. Bempedoic acid is a novel lipid-lowering drug recently approved for the treatment of dyslipidemia. This review describes the pharmacology of bempedoic acid and its clinical role in patients with dyslipidemia. Bempedoic acid, via its active coenzyme A (CoA) form, inhibits adenosine triphosphate (ATP)-citrate lyase, and reduces hepatic cholesterol synthesis through the mevalonate pathway. The reduction in plasma LDL-C by bempedoic acid is approximately 20%. In addition, this drug is able to lower the level of high-sensitivity C-reactive protein (hs-CRP) by 20%, which suggests anti-inflammatory activity. Bempedoic acid is well tolerated by the majority of patients. Possible common adverse drug reactions include upper respiratory tract infection, urinary tract infection and arthralgia. Serum creatinine and uric acid should be monitored since increased creatinine and hyperuricemia-associated new onset of gout and gout flares have been reported in patients taking bempedoic acid. Decreased hemoglobin levels and rare tendon ruptures have also been observed. Due to its efficacy and good safety profile, bempedoic acid might serve as a potential therapeutic alternative for the management of dyslipidemia.


Similar content being viewed by others
References
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65.
Romanelli RJ, Ito MK, Karalis DG, Huang HC, Iorga SR, Kam IW, et al. Statin utilization and low-density lipoprotein cholesterol in statin-treated patients with atherosclerotic cardiovascular disease: trends from a community-based health care delivery system, 2002–2016. J Clin Lipidol. 2020;14(3):305–14.
Kunlamas Y, Areepium N, Ariyachaipanich A, Bunditanukul K. Real-world effectiveness of high-versus moderate-intensity statin therapy in Thai patients with acute coronary syndrome and who had undergone primary percutaneous coronary intervention. J Pharm Pract. 2020;33(5):640–6.
Phan BA, Dayspring TD, Toth PP. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag. 2012;8:415–27.
Hess CN, Low Wang CC, Hiatt WR. PCSK9 inhibitors: mechanisms of action, metabolic effects, and clinical outcomes. Annu Rev Med. 2018;29(69):133–45.
Markham A. Bempedoic acid: first approval. Drugs. 2020;80(7):747–53.
Wang Q, Jiang L, Wang J, Li S, Yu Y, You J, et al. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology. 2009;49(4):1166–75.
Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380(11):1033–42.
Ference BA, Ray KK, Nicholls SJ. Mendelian randomization study of ACLY and cardiovascular disease. Reply. N Engl J Med. 2020;383(7):e50.
Damask A, Paulding C, Baras A, Carey D, Abecasis GR. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2020;383(7):e50.
Holm H, Sulem P, Helgadottir A, Tragante V, Thornorleifsson G, Guethbjartsson D, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2020;383(7):e50.
Klarin D, O’Donnell CJ, Kathiresan S. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2020;383(7):e50.
Cramer CT, Goetz B, Hopson KL, Fici GJ, Ackermann RM, Brown SC, et al. Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J Lipid Res. 2004;45(7):1289–301.
Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134–51.
Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun. 2016;28(7):13457.
Mendoza-Oliva A, Zepeda A, Arias C. The complex actions of statins in brain and their relevance for Alzheimer’s disease treatment: an analytical review. Curr Alzheimer Res. 2014;11(9):817–33.
Feng X, Zhang L, Xu S, Shen AZ. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: an updated review. Prog Lipid Res. 2020;77:101006.
Samsoondar JP, Burke AC, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, et al. Prevention of diet-induced metabolic dysregulation, inflammation, and atherosclerosis in Ldlr(-/-) mice by treatment with the ATP-Citrate lyase inhibitor bempedoic acid. Arterioscler Thromb Vasc Biol. 2017;37(4):647–56.
Ballantyne CM, Bays H, Catapano AL, Goldberg A, Ray KK, Saseen JJ. Role of bempedoic acid in clinical practice. Cardiovasc Drugs Ther. 2021;5:853–64.
Zhao S, Torres A, Henry RA, Trefely S, Wallace M, Lee JV, et al. ATP-citrate lyase controls a glucose-to-acetate metabolic switch. Cell Rep. 2016;17(4):1037–52.
Burke AC, Telford DE, Sutherland BG, Edwards JY, Sawyez CG, Barrett PHR, et al. Bempedoic acid lowers low-density lipoprotein cholesterol and attenuates atherosclerosis in low-density lipoprotein receptor-deficient (LDLR(+/-) and LDLR(-/-)) Yucatan miniature pigs. Arterioscler Thromb Vasc Biol. 2018;38(5):1178–90.
Dominguez M, Brune B, Namgaladze D. Exploring the role of ATP-citrate lyase in the immune system. Front Immunol. 2021;12:632526.
Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440(1):105–11.
Baardman J, Verberk SGS, van der Velden S, Gijbels MJJ, van Roomen C, Sluimer JC, et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat Commun. 2020;11(1):6296.
Filippov S, Pinkosky SL, Lister RJ, Pawloski C, Hanselman JC, Cramer CT, et al. ETC-1002 regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMPK. J Lipid Res. 2013;54(8):2095–108.
Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–32.
Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 2019;322(18):1780–8.
Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203.
Wang X, Zhang Y, Tan H, Wang P, Zha X, Chong W, et al. Efficacy and safety of bempedoic acid for prevention of cardiovascular events and diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19(1):128.
Nicholls S, Lincoff AM, Bays HE, Cho L, Grobbee DE, Kastelein JJ, et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2020;24(235):104–12.
Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Management of Dyslipidemia and Prevention of Cardiovascular Disease Algorithm—2020 executive summary. Endocr Pract. 2020;26(10):1196–224.
Bays HE, Banach M, Catapano AL, Duell PB, Gotto AM Jr, Laufs U, et al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol. 2020;14(5):649-59 e6.
Backes JM, Hilleman DE. New and emerging lipid-lowering therapy. Future Cardiol. 2021. https://doi.org/10.2217/fca-2020-0217.
Kunze A, Huwyler J, Camenisch G, Poller B. Prediction of organic anion-transporting polypeptide 1B1- and 1B3-mediated hepatic uptake of statins based on transporter protein expression and activity data. Drug Metab Dispos. 2014;42(9):1514–21.
Lalwani ND, Hanselman JC, MacDougall DE, Sterling LR, Cramer CT. Complementary low-density lipoprotein-cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC-1002) to high-dose atorvastatin background therapy in hypercholesterolemic patients: a randomized placebo-controlled trial. J Clin Lipidol. 2019;13(4):568–79.
Delevry D, Gupta EK. Bempedoic acid: review of a novel therapy in lipid management. Am J Health Syst Pharm. 2021;78(2):95–104.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding support for this work.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SW designed the concept and structure of the review. SW reviewed the pharmacological properties and clinical studies. WS assisted with the interpretation of the preclinical and clinical studies. SW drafted the manuscript. SW and WS critically reviewed and approved the final version of the manuscript.
Rights and permissions
About this article
Cite this article
Wichaiyo, S., Supharattanasitthi, W. Bempedoic Acid: A New Non-statin Drug for the Treatment of Dyslipidemia. Clin Drug Investig 41, 843–851 (2021). https://doi.org/10.1007/s40261-021-01075-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01075-w