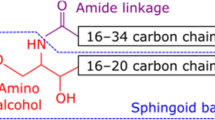
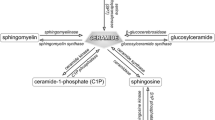
Abstract
Ceramides are a class of sphingolipid that is the backbone structure for all sphingolipids, such as glycosphingolipids and phosphosphingolipids. While being a minor constituent of cellular membranes, ceramides are the major lipid component (along with cholesterol, free fatty acid, and other minor components) of the intercellular spaces of stratum corneum that forms the epidermal permeability barrier. These stratum corneum ceramides consist of unique heterogenous molecular species that have only been identified in terrestrial mammals. Alterations of ceramide molecular profiles are characterized in skin diseases associated with compromised permeability barrier functions, such as atopic dermatitis, psoriasis and xerosis. In addition, hereditary abnormalities of some ichthyoses are associated with an epidermal unique ceramide species, omega-O-acylceramide. Ceramides also serve as lipid modulators to regulate cellular functions, including cell cycle arrest, differentiation, and apoptosis, and it has been demonstrated that changes in ceramide metabolism also cause certain diseases. In addition, ceramide metabolites, sphingoid bases, sphingoid base-1-phosphate and ceramide-1-phosphate are also lipid mediators that regulate cellular functions. In this review article, we describe diverse physiological and pathological roles of ceramides and their metabolites in epidermal permeability barrier function, epidermal cell proliferation and differentiation, immunity, and cutaneous diseases. Finally, we summarize the utilization of ceramides as therapy to treat cutaneous disease.







Similar content being viewed by others
References
Thudicum JLW. A treatise on the chemical constitution of brain. London: Bailliere, Tindall and Cox; 1884.
Uchida Y. Ceramide signaling in mammalian epidermis. Biochim Biophys Acta. 2014;1841:453–62.
Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metab. 2019;1:1051–8.
Albeituni S, Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv Exp Med Biol. 2019;1161:169–91.
Colombini M. Ceramide channels. Adv Exp Med Biol. 2019;1159:33–48.
Presa N, Gomez-Larrauri A, Dominguez-Herrera A, Trueba M, Gomez-Munoz A. Novel signaling aspects of ceramide 1-phosphate. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158630.
Berwick ML, Dudley BA, Maus K, Chalfant CE. The Role of ceramide 1-phosphate in inflammation, cellular proliferation, and wound healing. Adv Exp Med Biol. 2019;1159:65–77.
Schmitt T, Neubert RHH. State of the art in stratum corneum research: the biophysical properties of ceramides. Chem Phys Lipids. 2018;216:91–103.
Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87.
Uchida Y, Hamanaka S. Stratum corneum ceramides: function, origins, and therapeutic applications. In: Elias PM, Feingold KR, editors. Skin barrier. New York: Taylor & Francis; 2006. p. 43–65.
van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313.
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–51.
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, et al. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–76.
Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008;8:299–305.
Thiele JJ. Oxidative targets in the stratum corneum. A new basis for antioxidative strategies. Skin Pharmacol Appl Skin Physiol. 2001;14(Suppl 1):87–91.
Mildner M, Jin J, Eckhart L, Kezic S, Gruber F, Barresi C, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286–94.
Bow JR, Sonoki Y, Uchiyama M, Shimizu E, Tanaka K, Dauskardt RH. Lipid loss increases stratum corneum stress and drying rates. Skin Pharmacol Physiol. 2020;33:180–8.
Afshar M, Gallo RL. Innate immune defense system of the skin. Vet Dermatol. 2013;24:32-8.e8-9.
Uchida Y, Park K. Stratum corneum. In: Kabashima K, editor. Immunology of the skin. Tokyo: Springer; 2016. p. 15–30.
Uchida Y, Park K. Anti-microbial peptides in skin barrier functions. J Skin Barrier Res. 2013;15:1–8.
Yokouchi M, Kubo A. Maintenance of tight junction barrier integrity in cell turnover and skin diseases. Exp Dermatol. 2018;27:876–83.
van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23:45–52.
Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94.
Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br J Dermatol. 2005;152:426–34.
Yamamoto H, Hattori M, Chamulitrat W, Ohno Y, Kihara A. Skin permeability barrier formation by the ichthyosis-causative gene FATP4 through formation of the barrier lipid omega-O-acylceramide. Proc Natl Acad Sci USA. 2020;117:2914–22.
Ohno Y, Kamiyama N, Nakamichi S, Kihara A. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid omega-O-acylceramide. Nat Commun. 2017;8:14610.
Ohno Y, Nakamichi S, Ohkuni A, Kamiyama N, Naoe A, Tsujimura H, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci USA. 2015;112:7707–12.
Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA. 2010;107:18439–44.
Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, et al. Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation. J Biol Chem. 2007;282:13211–9.
Vasireddy V, Uchida Y, Salem N Jr, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (>=C28) and the unique {omega}-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–82.
Uchida Y, Cho Y, Moradian S, Kim J, Nakajima K, Crumrine D, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of dorfman-chanarin syndrome. J Invest Dermatol. 2010;130:2497–9.
Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608.
Hansen HS, Jensen B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim Biophys Acta. 1985;834:357–63.
McIntosh TJ, Stewart ME, Downing DT. X-ray diffraction analysis of isolated skin lipids: reconstitution of intercellular lipid domains. Biochemistry. 1996;35:3649–53.
Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ijzerman AP, Ponec M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res. 1998;39:186–96.
Opalka L, Kovacik A, Pullmannova P, Maixner J, Vavrova K. Effects of omega-O-acylceramide structures and concentrations in healthy and diseased skin barrier lipid membrane models. J Lipid Res. 2020;61:219–28.
de Jager M, Gooris G, Ponec M, Bouwstra J. Acylceramide head group architecture affects lipid organization in synthetic ceramide mixtures. J Invest Dermatol. 2004;123:911–6.
Schmitt T, Neubert RHH. State of the art in stratum corneum research. Part II: hypothetical stratum corneum lipid matrix models. Skin Pharmacol Physiol. 2020;33:213–30.
Nakazawa H, Imai T, Hatta I, Sakai S, Inoue S, Kato S. Low-flux electron diffraction study for the intercellular lipid organization on a human corneocyte. Biochim Biophys Acta. 2013;1828:1424–31.
Schmitt T, Gupta R, Lange S, Sonnenberger S, Dobner B, Hauss T, et al. Impact of the ceramide subspecies on the nanostructure of stratum corneum lipids using neutron scattering and molecular dynamics simulations. Part I: impact of CER[NS]. Chem Phys Lipids. 2018;214:58–68.
Schmitt T, Lange S, Sonnenberger S, Dobner B, Deme B, Langner A, et al. The long periodicity phase (LPP) controversy part I: The influence of a natural-like ratio of the CER[EOS] analogue [EOS]-br in a CER[NP]/[AP] based stratum corneum modelling system: a neutron diffraction study. Biochim Biophys Acta Biomembr. 2019;1861:306–15.
Kawana M, Miyamoto M, Ohno Y, Kihara A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J Lipid Res. 2020;61:884–95.
Uchida Y, Iwamori M, Nagai Y. Distinct differences in lipid composition between epidermis and dermis from footpad and dorsal skin of guinea pigs. Jpn J Exp Med. 1988;58:153–61.
Wertz PW, Downing DT. Ceramides of pig epidermis: structure determination. J Lipid Res. 1983;24:759–65.
Angelbeck-Schulze M, Stahl J, Brodesser S, Rohn K, Naim H, Hewicker-Trautwein M, et al. Comparison of three different sampling methods for canine skin lipids. Vet Dermatol. 2013;24:233-e51.
Akiyama M. Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis. J Dermatol Sci. 2017;88:3–9.
Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, et al. Formation and functions of the corneocyte lipid envelope (CLE). Biochim Biophys Acta. 2014;1841:314–8.
Taniguchi M, Okazaki T. Ceramide/sphingomyelin rheostat regulated by sphingomyelin synthases and chronic diseases in murine models. J Lipid Atheroscler. 2020;9:380–405.
Summers SA. Ceramides: nutrient signals that drive hepatosteatosis. J Lipid Atheroscler. 2020;9:50–65.
Kim S, Hong I, Hwang JS, Choi JK, Rho HS, Kim DH, et al. Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin. Mol Med. 2006;12:17–24.
Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33:752–62.
Shin KO, Kim KP, Cho Y, Kang MK, Kang YH, Lee YM, et al. Both sphingosine kinase 1 and 2 coordinately regulate cathelicidin antimicrobial peptide production during keratinocyte differentiation. J Invest Dermatol. 2019;139:492–4.
Uchida Y, Iwamori M, Nagai Y. Activation of keratinization of keratinocytes from fetal rat skin with N-(O-linoleoyl) omega-hydroxy fatty acyl sphingosyl glucose (lipokeratinogenoside) as a marker of epidermis. Biochem Biophys Res Commun. 1990;170:162–8.
Uchida Y, Ogawa T, Iwamori M, Nagai Y. Enhancement of keratin synthesis induced by lipokeratinogenoside, N-(O-linoleoyl)-omega-hydroxy fatty acyl sphingosyl glucose, in association with alteration of the intracellular Ca(2+)-content and protein kinase in cultured keratinocytes (FRSK). J Biochem. 1991;109:462–5.
Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56.
Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–901.
Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7.
Taniguchi M, Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim Biophys Acta. 2014;1841:692–703.
D’Angelo G, Moorthi S, Luberto C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv Cancer Res. 2018;140:61–96.
Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–51.
Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–23.
Heinrich M, Wickel M, Winoto-Morbach S, Schneider-Brachert W, Weber T, Brunner J, et al. Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol. 2000;477:305–15.
Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–95.
Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–8.
Jiang YJ, Kim P, Uchida Y, Elias PM, Bikle DD, Grunfeld C, et al. Ceramides stimulate caspase-14 expression in human keratinocytes. Exp Dermatol. 2013;22:113–8.
Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–41.
Sigruener A, Tarabin V, Paragh G, Liebisch G, Koehler T, Farwick M, et al. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp Dermatol. 2013;22:677–9.
Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–8.
Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–20.
Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596–608.
Wang Z, Zheng Y, Wang F, Zhong J, Zhao T, Xie Q, et al. Mfsd2a and Spns2 are essential for sphingosine-1-phosphate transport in the formation and maintenance of the blood-brain barrier. Sci Adv. 2020;6:8627.
Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, et al. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–9.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–7.
Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci USA. 2016;113:E1334-42.
Clarke CJ, Wu BX, Hannun YA. The neutral sphingomyelinase family: identifying biochemical connections. Adv Enzyme Regul. 2011;51:51–8.
Goni FM, Alonso A. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 2002;531:38–46.
Collenburg L, Beyersdorf N, Wiese T, Arenz C, Saied EM, Becker-Flegler KA, et al. The activity of the neutral sphingomyelinase is important in T cell recruitment and directional migration. Front Immunol. 2017;8:1007.
Bai A, Kokkotou E, Zheng Y, Robson SC. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015;6:e1828.
De Lira MN, Raman SJ, Schulze A, Schneider-Schaulies S, Avota E. Neutral sphingomyelinase-2 (NSM 2) controls T cell metabolic homeostasis and reprogramming during activation. Front Mol Biosci. 2020;7:217.
Reines I, Kietzmann M, Mischke R, Tschernig T, Luth A, Kleuser B, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:1954–62.
Japtok L, Schaper K, Baumer W, Radeke HH, Jeong SK, Kleuser B. Sphingosine 1-phosphate modulates antigen capture by murine langerhans cells via the S1P2 receptor subtype. PLoS ONE. 2012;7:e49427.
Kim YI, Park K, Kim JY, Seo HS, Shin KO, Lee YM, et al. An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating beta-defensin production. Mol Cell Biol. 2014;34:4368–78.
Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–30.
Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101.
Melnik B, Hollmann J, Plewig G. Decreased stratum corneum ceramides in atopic individuals—a pathobiochemical factor in xerosis? Br J Dermatol. 1988;119:547–9.
Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–23.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6.
Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30.
Boer DEC, van Smeden J, Al-Khakany H, Melnik E, van Dijk R, Absalah S, et al. Skin of atopic dermatitis patients shows disturbed beta-glucocerebrosidase and acid sphingomyelinase activity that relates to changes in stratum corneum lipid composition. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158673.
Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–66.
Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–78.
McLean WH. Filaggrin failure—from ichthyosis vulgaris to atopic eczema and beyond. Br J Dermatol. 2016;175(Suppl 2):4–7.
Fartasch M, Bassukas ID, Diepgen TL. Disturbed extruding mechanism of lamellar bodies in dry non-eczematous skin of atopics. Br J Dermatol. 1992;127:221–7.
Chermprapai S, Broere F, Gooris G, Schlotter YM, Rutten V, Bouwstra JA. Altered lipid properties of the stratum corneum in canine atopic dermatitis. Biochim Biophys Acta Biomembr. 2018;1860:526–33.
Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710–7.
Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–74.
Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–92.
Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, et al. Interferon-gamma decreases ceramides with long-chain fatty acids: possible involvement in atopic dermatitis and psoriasis. J Invest Dermatol. 2014;134:712–8.
Sawada E, Yoshida N, Sugiura A, Imokawa G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: an implication for the disrupted barrier mechanism in atopic dermatitis. J Dermatol Sci. 2012;68:25–35.
Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000;115:406–13.
Oizumi A, Nakayama H, Okino N, Iwahara C, Kina K, Matsumoto R, et al. Pseudomonas-derived ceramidase induces production of inflammatory mediators from human keratinocytes via sphingosine-1-phosphate. PLoS ONE. 2014;9:89402.
van Smeden J, Janssens M, Boiten WA, van Drongelen V, Furio L, Vreeken RJ, et al. Intercellular skin barrier lipid composition and organization in netherton syndrome patients. J Invest Dermatol. 2014;134:1238–45.
van Smeden J, Al-Khakany H, Wang Y, Visscher D, Stephens N, Absalah S, et al. Skin barrier lipid enzyme activity in Netherton patients is associated with protease activity and ceramide abnormalities. J Lipid Res. 2020;61:859–69.
Yokose U, Ishikawa J, Morokuma Y, Naoe A, Inoue Y, Yasuda Y, et al. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020;20:6.
Cho Y, Lew BL, Seong K, Kim NI. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 2004;19:859–63.
Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89:745–50.
Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, et al. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15:1259–63.
Carrie L, Virazels M, Dufau C, Montfort A, Levade T, Segui B, et al. New insights into the role of sphingolipid metabolism in melanoma. Cells. 2020;9:1967.
Takeichi T, Torrelo A, Lee JYW, Ohno Y, Lozano ML, Kihara A, et al. Biallelic mutations in KDSR disrupt ceramide synthesis and result in a spectrum of keratinization disorders associated with thrombocytopenia. J Invest Dermatol. 2017;137:2344–53.
Boyden LM, Vincent NG, Zhou J, Hu R, Craiglow BG, Bayliss SJ, et al. Mutations in KDSR cause recessive progressive symmetric erythrokeratoderma. Am J Hum Genet. 2017;100:978–84.
Lin CL, Xu R, Yi JK, Li F, Chen J, Jones EC, et al. Alkaline ceramidase 1 protects mice from premature hair loss by maintaining the homeostasis of hair follicle stem cells. Stem Cell Reports. 2017;9:1488–500.
Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8:1106–11.
Novotny J, Hrabalek A, Vavrova K. Synthesis and structure-activity relationships of skin ceramides. Curr Med Chem. 2010;17:2301–24.
Kaneko T, Tanaka T, Nagase M. Agent for protecting skin and hair moisture. US Patent 635532. 2002.
Berkers T, Visscher D, Gooris GS, Bouwstra JA. Topically applied ceramides interact with the stratum corneum lipid matrix in compromised ex vivo skin. Pharm Res. 2018;35:48.
Sahle FF, Metz H, Wohlrab J, Neubert RH. Polyglycerol fatty acid ester surfactant-based microemulsions for targeted delivery of ceramide AP into the stratum corneum: formulation, characterisation, in vitro release and penetration investigation. Eur J Pharm Biopharm. 2012;82:139–50.
Tessema EN, Gebre-Mariam T, Frolov A, Wohlrab J, Neubert RHH. Development and validation of LC/APCI-MS method for the quantification of oat ceramides in skin permeation studies. Anal Bioanal Chem. 2018;410:4775–85.
Kovacik A, Pullmannova P, Maixner J, Vavrova K. Effects of ceramide and dihydroceramide stereochemistry at c-3 on the phase behavior and permeability of skin lipid membranes. Langmuir. 2018;34:521–9.
Tessema EN, Gebre-Mariam T, Neubert RHH, Wohlrab J. Potential applications of phyto-derived ceramides in improving epidermal barrier function. Skin Pharmacol Physiol. 2017;30:115–38.
Morifuji M. The beneficial role of functional food components in mitigating ultraviolet-induced skin damage. Exp Dermatol. 2019;28(Suppl 1):28–31.
Vollmer DL, West VA, Lephart ED. Enhancing Skin Health: By Oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–49.
Merrill AH Jr. Characterization of serine palmitoyltransferase activity in chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–91.
Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem. 2006;281:37275–81.
Beeler T, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, et al. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Delta mutant. J Biol Chem. 1998;273:30688–94.
Mizutani Y, Kihara A, Igarashi Y. Identification of the human sphingolipid C4-hydroxylase, hDES2, and its up-regulation during keratinocyte differentiation. FEBS lett. 2004;563:93–7.
Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem. 2004;279:49243–50.
Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–56.
Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, et al. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47:1063–70.
Sassa T, Ohno Y, Suzuki S, Nomura T, Nishioka C, Kashiwagi T, et al. Impaired epidermal permeability barrier in mice lacking elovl1, the gene responsible for very-long-chain fatty acid production. Mol Cell Biol. 2013;33:2787–96.
Lin MH, Hsu FF, Crumrine D, Meyer J, Elias PM, Miner JH. Fatty acid transport protein 4 is required for incorporation of saturated ultralong-chain fatty acids into epidermal ceramides and monoacylglycerols. Sci Rep. 2019;9:13254.
Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–80.
Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J Biol Chem. 2010;285:7300–11.
Hirabayashi T, Anjo T, Kaneko A, Senoo Y, Shibata A, Takama H, et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat Commun. 2017;8:14609.
Zhou J, Saba JD. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun. 1998;242:502–7.
Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, et al. Ceramide kinase, a novel lipid kinase Molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–300.
Acknowledgements
We thank Ms Joan Wakefield for superb editorial assistance (Northern California Institute for Research and Education, San Francisco Veterans Affairs Health Care System, and the University of California San Francisco). We acknowledge the support of the Medical Research Services of the Veterans Affairs Medical Center, San Francisco.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by the National Institutes of Health Grants R01 AR062025 (to YU), and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B7050504), the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program (reference number P0014701) for The Industries of Economic Cooperation Region and Main Research Program (E0210600-01) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT (to KP). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
Authors state no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Both YU and KP researched, wrote and re-wrote this manuscript.
Rights and permissions
About this article
Cite this article
Uchida, Y., Park, K. Ceramides in Skin Health and Disease: An Update. Am J Clin Dermatol 22, 853–866 (2021). https://doi.org/10.1007/s40257-021-00619-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00619-2