Abstract
Background
Scalp psoriasis is commonly the initial presentation of psoriasis, and almost 80 % of patients with psoriasis will eventually experience it.
Objective
Although several systematic reviews and guidelines exist, an up-to-date evidence-based review including more recent progress on the use of biologics and new oral small molecules was timely.
Methods
Of the 475 studies initially retrieved from PubMed and the 845 from Embase (up to May 2016), this review includes 27 clinical trials, four papers reporting pooled analyses of other clinical trials, ten open-label trials, one case series, and two case reports after excluding non-English literature.
Results
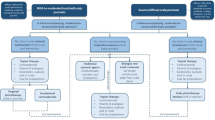
To our knowledge, few randomized controlled trials (RCTs) are conducted specifically in scalp psoriasis. Topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is well tolerated and more effective than either of its individual components. Localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but we found no published RCTs evaluating these agents specifically in scalp psoriasis. Biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis.
Conclusions
More controlled studies are needed for an evidence-based approach to scalp psoriasis.

Similar content being viewed by others
References
Chan CS, Van Voorhees AS, Lebwohl MG, Korman NJ, Young M, Bebo BF Jr, et al. Treatment of severe scalp psoriasis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2009;60(6):962–71.
Mason AR, Mason JM, Cork MJ, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. Br J Dermatol. 2013;169(3):519–27.
Osier E, Gomez B, Eichenfield LF. Adolescent scalp psoriasis: update on topical combination therapy. J Clin Aesthet Dermatol. 2015;8(7):43–7.
Papp K, Berth-Jones J, Kragballe K, Wozel G, de la Brassinne M. Scalp psoriasis: a review of current topical treatment options. J Eur Acad Dermatol Venereol. 2007;21(9):1151–60.
Schlager JG, Rosumeck S, Werner RN, Jacobs A, Schmitt J, Schlager C, et al. Topical treatments for scalp psoriasis. Cochrane Database Syst Rev. 2016;2:CD009687.
Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trueb RM. Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23(12):1435–44.
Feldman SR, Housman TS. Patients’ vehicle preference for corticosteroid treatments of scalp psoriasis. Am J Clin Dermatol. 2003;4(4):221–4.
Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(Suppl. 1):S39–49.
Abraham A, Roga G. Topical steroid-damaged skin. Indian J Dermatol. 2014;59(5):456–9.
Turpeinen M. Adrenocortical response to adrenocorticotropic hormone in relation to duration of topical therapy and percutaneous absorption of hydrocortisone in children with dermatitis. Eur J Pediatr. 1989;148(8):729–31.
Lassus A. Local treatment of psoriasis of the scalp with clobetasol propionate and betamethasone-17,21-dipropionate: a double-blind comparison. Curr Med Res Opin. 1976;4(5):365–7.
Jarratt M, Breneman D, Gottlieb AB, Poulin Y, Liu Y, Foley V. Clobetasol propionate shampoo 0.05 %: a new option to treat patients with moderate to severe scalp psoriasis. J Drugs Dermatol. 2004;3(4):367–73.
Andres P, Poncet M, Farzaneh S, Soto P. Short-term safety assessment of clobetasol propionate 0.05 % shampoo: hypothalamic-pituitary adrenal axis suppression, atrophogenicity, and ocular safety in subjects with scalp psoriasis. J Drugs Dermatol. 2006;5(4):328–32.
Reygagne P, Mrowietz U, Decroix J, de Waard-van der Spek FB, Acebes LO, Figueiredo A, et al. Clobetasol propionate shampoo 0.05 % and calcipotriol solution 0.005 %: a randomized comparison of efficacy and safety in subjects with scalp psoriasis. J Dermatol Treat. 2005;16(1):31–6.
Griffiths CE, Finlay AY, Fleming CJ, Barker JN, Mizzi F, Arsonnaud S. A randomized, investigator-masked clinical evaluation of the efficacy and safety of clobetasol propionate 0.05 % shampoo and tar blend 1 % shampoo in the treatment of moderate to severe scalp psoriasis. J Dermatol Treat. 2006;17(2):90–5.
Willis I, Cornell RC, Penneys NS, Zaias N. Multicenter study comparing 0.05 % gel formulations of desoximetasone and fluocinonide in patients with scalp psoriasis. Clin Ther. 1986;8(3):275–82.
Andreassi L, Giannetti A. Milani M; scale investigators group. Efficacy of betamethasone valerate mousse in comparison with standard therapies on scalp psoriasis: an open, multicentre, randomized, controlled, cross-over study on 241 patients. Br J Dermatol. 2003;148(1):134–8.
Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12 %: a novel vehicle with enhanced delivery and efficacy. Int J Dermatol. 1999;38(8):628–32.
Klaber MR, Hutchinson PE, Pedvis-Leftick A, Kragballe K, Reunala TL, Van de Kerkhof PC, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17-valerate solution (1 mg/ml) in the treatment of scalp psoriasis. Br J Dermatol. 1994;131(5):678–83.
Pauporte M, Maibach H, Lowe N, Pugliese M, Friedman DJ, Mendelsohn H, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatol Treat. 2004;15(6):360–4.
Green C, Ganpule M, Harris D, Kavanagh G, Kennedy C, Mallett R, et al. Comparative effects of calcipotriol (MC 903) solution and placebo (vehicle of MC 903) in the treatment of psoriasis of the scalp. Br J Dermatol. 1994;130(4):483–7.
Okubo Y, Natsume S, Usui K, Muro M, Tsuboi R. Combination therapy using maxacalcitol and corticosteroid lotions preliminary to monotherapy with maxacalcitol lotion for scalp psoriasis. J Dermatol Treat. 2014;25(1):34–7.
Tyring S, Mendoza N, Appell M, Bibby A, Foster R, Hamilton T, et al. A calcipotriene/betamethasone dipropionate two-compound scalp formulation in the treatment of scalppsoriasis in Hispanic/Latino and Black/African American patients: results of the randomized, 8-week, double-blind phase of a clinical trial. Int J Dermatol. 2010;49(11):1328–33.
Buckley C, Hoffmann V, Shapiro J, Saari S, Cambazard F, Milsgaard M. Calcipotriol plus betamethasone dipropionate scalp formulation is effective and well tolerated in the treatment of scalp psoriasis: a phase II study. Dermatology. 2008;217(2):107–13.
Kragballe K, Hoffmann V, Ortonne JP, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. Br J Dermatol. 2009;161(1):159–66.
Ma L, Yang Q, Yang H, Wang G, Zheng M, Hao F, et al. Calcipotriol plus betamethasone dipropionate gel compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized, controlled trial investigating efficacy and safety in a Chinese population. Int J Dermatol. 2016;55(1):106–13.
Luger TA, Cambazard F, Larsen FG, Bourcier M, Gupta G, Clonier F, et al. A study of the safety and efficacy of calcipotriol and betamethasone dipropionate scalp formulation in the long-term management of scalp psoriasis. Dermatology. 2008;217(4):321–8.
Jemec GB, Ganslandt C, Ortonne JP, Poulin Y, Burden AD, de Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double-blind, controlled trial. J Am Acad Dermatol. 2008;59(3):455–63.
van de Kerkhof PC, Hoffmann V, Anstey A, Barnes L, Bolduc C, Reich K, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: arandomized, double-blind, controlled trial. Br J Dermatol. 2009;160(1):170–6.
Gooderham M, Debarre JM, Keddy-Grant J, Xu Z, Kurvits M, Goodfield M. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12–17 years of age. Br J Dermatol. 2015;171(6):1470–7.
Eichenfield LF, Ganslandt C, Kurvits M, Schlessinger J. Safety and efficacy of calcipotriene plus betamethasone dipropionate topical suspension in the treatment of extensive scalp psoriasis in adolescents ages 12 to 17 years. Pediatr Dermatol. 2015;32(1):28–35.
Oostveen AM, de Jong EM, Donders AR, van de Kerkhof PC, Seyger MM. Treatment of paediatric scalp psoriasis with calcipotriene/betamethasone dipropionate scalp formulation: effectiveness, safety and influence on children’s quality of life in daily practice. J Eur Acad Dermatol Venereol. 2015;29(6):1193–7.
Gual A, Pau-Charles I, Molin S. Topical treatment for scalp psoriasis: Comparison of patient preference, quality of life and efficacy for non-alcoholic mometasone emulsion versus calcipotriol/betamethasone gel in daily clinical practice. J Dermatol Treat. 2016;27(3):228–34.
Kragballe K, van de Kerkhof P. Pooled safety analysis of calcipotriol plus betamethasone dipropionate gel for the treatment of psoriasis on the body and scalp. J Eur Acad Dermatol Venereol. 2014;28(Suppl. 2):10–21.
Saraceno R, Camplone G, D’Agostino M, De Simone C, Di Cesare A, Filosa G, et al. Efficacy and maintenance strategies of two-compound formulation calcipotriol and betamethasonedipropionate gel (xamiol® gel) in the treatment of scalp psoriasis: results from a study in 885 patients. J Dermatol Treat. 2014;25(1):30–3.
Arnold WP. Tar. Clin Dermatol. 1997;15(5):739–44.
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3: guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–59.
Cunliffe WJ, Dodman B. A comparison of tar liquid and cetrimide shampoo in the management of psoriasis of the scalp. Br J Clin Pract. 1974;28(9):314–5.
Kircik L. Salicylic Acid 6 % in an ammonium lactate emollient foam vehicle in the treatment of mild-to-moderate scalp psoriasis. J Drugs Dermatol. 2011;10(3):270–3.
Shokeen D, O’Neill JL, Taheri A, Feldman SR. Are topical keratolytic agents needed in the treatment of scalp psoriasis? Dermatol Online J. 2014;20(3). http://escholarship.org/uc/item/7g56436q.
Hovding G. Treatment of psoriasis of the scalp with betamethasone 17, 21-dipropionate plus salicylic acid lotion (‘diprosalic’). Pharmatherapeutica. 1981;3(1):61–6.
Roberts DL, Marshall R, Marks R. Detection of the action of salicylic acid on the normal stratum corneum. Br J Dermatol. 1980;103(2):191–6.
Solomon AE, Lowe NJ. Percutaneous absorption in experimental epidermal disease. Br J Dermatol. 1979;100(6):717–22.
Hillstrom L. Comparison of topical treatment with desoxymethasone solution 0.25 % with salicylic acid 1 % and betamethasone valerate solution 0.1 % in patients with psoriasis of the scalp. J Int Med Res. 1984;12(3):170–3.
Hillstrom L, Pettersson L, Svensson L. Comparison of betamethasone dipropionate lotion with salicylic acid (diprosalic) and clobetasol propionate lotion (dermovate) in the treatment of psoriasis of the scalp. J Int Med Res. 1982;10(6):419–22.
Elie R, Durocher LP, Kavalec EC. Effect of salicylic acid on the activity of betamethasone-17,21-dipropionate in the treatment of erythematous squamous dermatoses. J Int Med Res. 1983;11(2):108–12.
Fartasch M, Teal J, Menon GK. Mode of action of glycolic acid on human stratum corneum: ultrastructural and functional evaluation of the epidermal barrier. Arch Dermatol Res. 1997;289(7):404–9.
Kostarelos K, Teknetzis A, Lefaki I, Ioannides D, Minas A. Double-blind clinical study reveals synergistic action between alpha-hydroxy acid and betamethasone lotions towards topical treatment of scalp psoriasis. J Eur Acad Dermatol Venereol. 2000;14(1):5–9.
Kemény L, Ruzicka T, Braun-Falco O. Dithranol: a review of the mechanism of action in the treatment of psoriasis vulgaris. Skin Pharmacol. 1990;3(1):1–20.
Wulff-Woesten A, Ohlendorf D, Henz BM, Haas N. Dithranol in an emulsifying oil base (bio-wash-oil) for the treatment of psoriasis of the scalp. Skin Pharmacol Physiol. 2004;17(2):91–7.
Gollnick HP, Finzi AF, Marks R, Barker JN, Jansen C, Revuz J, et al. Optimising the use of tazarotene in clinical practice: consensus statement from the European advisory panel for tazarotene (zorac TM). Dermatology. 1999;199(1):40–6.
Taneja A, Racette A, Gourgouliatos Z, Taylor CR. Broad-band UVB fiber-optic comb for the treatment of scalp psoriasis: a pilot study. Int J Dermatol. 2004;43(6):462–7.
Gattu S, Rashid RM, Wu JJ. 308-nm excimer laser in psoriasis vulgaris, scalp psoriasis, and palmoplantar psoriasis. J Eur Acad Dermatol Venereol. 2009;23(1):36–41.
Morison WL, Atkinson DF, Werthman L. Effective treatment of scalp psoriasis using the excimer (308 nm) laser. Photodermatol Photoimmunol Photomed. 2006;22(4):181–3.
Lindelöf B. Grenz ray therapy in dermatology. An experimental, clinical and epidemiological study. Acta Derm Venereol Suppl (Stockh). 1987;132:1–67.
Lindelöf B, Johannesson A. Psoriasis of the scalp treated with grenz rays or topical corticosteroid combined with grenz rays. a comparative randomized trial. Br J Dermatol. 1988;119(2):241–4.
Farr PM, Krause LB, Marks JM, Shuster S. Response of scalp psoriasis to oral ketoconazole. Lancet. 1985;2(8461):921–2.
Faergemann J, Diehl U, Bergfelt L, Brodd A, Edmar B, Hersle K, et al. Scalp psoriasis: synergy between the Malassezia yeasts and skin irritation due to calcipotriol. Acta Derm Venereol. 2003;83(6):438–41.
Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67(1):86–92.
Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR, et al. Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from arandomized, double-blind, placebo-controlled study of etanercept. J Eur Acad Dermatol Venereol. 2013;27(1):125–8.
Gottlieb AB. Infliximab for psoriasis. J Am Acad Dermatol. 2003;49(Suppl. 2):S112–7.
Torii H, Sato N, Yoshinari T, Nakagawa H, Japanese infliximab study investigators. Dramatic impact of a psoriasis area and severity index 90 response on the quality of life in patients with psoriasis: an analysis of Japanese clinical trials of infliximab. J Dermatol. 2012;39(3):253–9.
Torii H, Nakagawa H, Japanese infliximab study investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. a randomized, double-blind, placebo controlled multicenter trial. J Dermatol Sci. 2010;59(1):40–9.
Torii H, Nakagawa H, Japanese infliximab study investigators. Long term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38(4):321–34.
Menter A, Reich K, Li S, Guzzo C. Consistency of infliximab response in different body regions for treatment of moderate to severe psoriasis: results from controlled clinical trials. J Am Acad Dermatol. 2008;58(Suppl. 2):AB120.
Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–74.
Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–e15.
Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–42.
Thaçi D, Unnebrink K, Sundaram M, Sood S, Yamaguchi Y. Adalimumab for the treatment of moderate to severe psoriasis: subanalysis of effects onscalp and nails in the BELIEVE study. J Eur Acad Dermatol Venereol. 2015;29(2):353–60.
Wang TS, Tsai TF. Safety and effectiveness of adalimumab in patients with moderate-to-severe psoriasis who had inadequate therapeutic response to prior etanercept. Dermatol Sinica. 2013;31(1):11–8.
Papadavid E, Ferra D, Koumaki D, Dalamaga M, Stamou C, Theodoropoulos K, et al. Ustekinumab induces fast response and maintenance of very severe refractory scalp psoriasis: results in two Greek patients from the psoriasis hospital-based clinic. Dermatology. 2014;228(2):107–11.
Di Cesare A, Fargnoli MC, Peris K. Rapid response of scalp psoriasis to ustekinumab. Eur J Dermatol. 2011;21(6):993–4.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38.
Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–93.
Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082–90.
Kircik L, Fowler J, Weiss J, Meng X, Guana A, Nyirady J. Efficacy of secukinumab in the treatment of moderate-to-severe plaque psoriasis on the head and neck: pooled analysis of phase 3 studies. Poster presented at: American academy of dermatology 73rd annual meeting, San Francisco, CA, March 20–24, 2015.
Langley RG, Rich P, Menter A, Krueger G, Goldblum O, Dutronc Y, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2015;29(9):1763–70.
Reich K, Lebwohl M, Romiti R. Impact of ixekizumab treatment on scalp psoriasis: results from the UNCOVER-2 trial. Poster presented at: American academy of dermatology 74th annual meeting, Washington, DC, March 4, 2016.
Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–90.
Rich P, Gooderham M, Bachelez H, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74(1):134–42.
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (ESTEEM 1). J Am Acad Dermatol. 2015;73(1):37–49.
Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99.
Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–61.
Bissonnette R, Iversen L, Sofen H, Griffiths CE, Foley P, Romiti R, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis:a randomized controlled trial. Br J Dermatol. 2015;172:1395–406.
Chen HH, Tseng MP, Tsai TF. An epidemiologic study of Taiwanese psoriatic patients in a single clinic. Dermatol Sinica. 2003;21(3):216–24.
Wang TS, Tsai TF. Intralesional therapy for psoriasis. J Dermatol Treat. 2013;24(5):340–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manscript.
Conflict of interest
Dr. Tsai has conducted clinical trials or received honoraria for serving as a consultant for Pfizer, Novartis, Celgene, Eli-Lilly, and Janssen-Cilag Pharmaceuticals and has received speaking fees from Abbvie. Dr. Wang has received speaking fees from Abbvie and Janssen-Cilag Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Wang, TS., Tsai, TF. Managing Scalp Psoriasis: An Evidence-Based Review. Am J Clin Dermatol 18, 17–43 (2017). https://doi.org/10.1007/s40257-016-0222-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-016-0222-4